August 2019 Issue
ISSN 2689-291X
ISSN 2689-291X
Challenging Images
Endocarditis of the Mitral Valve! TEE Superiority!
Description
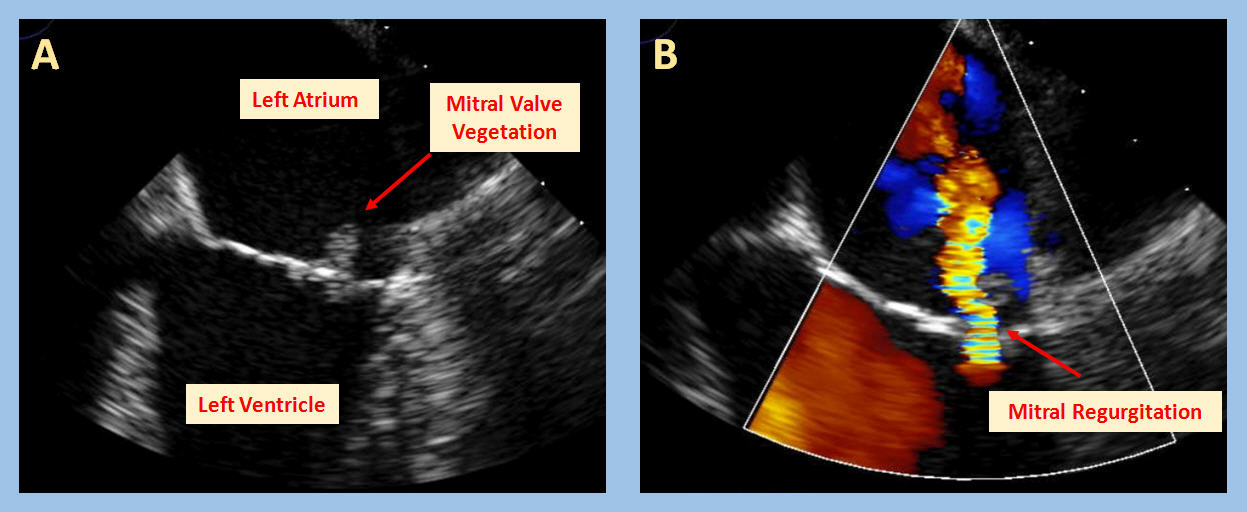
The transesophageal echocardiography images and videos show mild thickening of the anterior and posterior mitral valve leaflets, along with a highly mobile oscillating mass that can be visualized on the atrial aspect of the posterior mitral valve leaflet seen in Image A (red arrow) and video A. The mass is consistent with a vegetation, commonly diagnosed in patients with Infective Endocarditis (IE). Auscultation of a new murmur on cardiac physical examination, which reflects valvular damage due to the infection and subsequent valvular regurgitation, is a sign of endocarditis. This phenomenon is illustrated by the mild to moderate mitral valve regurgitation seen in Image B (video B).
IE has a relatively rare occurrence – a recent study found the 2011 incidence in the United States to be 15 per 100,000 persons, a slight increase from an incidence of 11 per 100,000 persons in 2000 [1]. The endothelium lining the valves of the healthy heart is designed to resist infective processes, but when the inner lining becomes damaged or a particularly virulent microorganism invades the bloodstream, infective endocarditis may occur, most commonly on the aortic or mitral valves. Endothelial disruption on the valvular surface may be caused by congenital heart defects, valvular dysfunction, the presence of a prosthetic valve, or prior infection, amongst other things. The damage of the endothelial cells leads to activation of the clotting cascade and the release of various inflammatory cytokines, eventually forming a thrombus, composed primarily of platelets and fibrin. The newly formed thrombus serves as a nidus for bacterial colonization during any period of transient bacteremia. This colonization results in the release of more inflammatory and clotting factors, creating a feed-forward type of mechanism that ultimately ends with the formation of a vegetation. Alternatively, infection can occur on a relatively normal valve in the setting of bacteremia with a more virulent organism, most commonly Staphylococcus aureus [2]. The diagnosis of endocarditis is largely guided by the use of the modified Duke criteria. Using this approach 2 major, 1 major and 3 minor, or 5 minor criteria are necessary to definitively make the diagnosis of infective endocarditis. The major criteria consist of specific parameters surrounding positive blood cultures as well as information supporting the involvement of the endocardium, most commonly provided through echocardiography, although other imaging modalities such as cardiac CT can be used [2,3]. Like any serious infection, endocarditis can be accompanied by numerous complications such as heart failure, abscess formation, and heart block, due to compromise of the cardiac conduction system in the setting of perivalvular extension [4]. Traditionally, IE is treated with parenteral antibiotics, although recent investigation has shown that transitioning to oral antibiotics in clinically stable patients may be acceptable [5]. Surgical intervention may be indicated in certain scenarios including a large vegetation (typically >10 mm) which carries a high risk for embolization, a resistant and progressive infection or, most commonly, in the event of severe valvular dysfunction [2,3]. The mortality rate associated with IE has shown minimal improvement throughout the years, remaining close to 30% in most cases. However, rates vary from case to case and differ based on the location, the infectious organism, the acuity, and associated comorbid conditions [3].
References:
Authors:
Morgan Roberts, B.S.
Medical Student
University of South Alabama
Mobile, AL
Galen Garriga, B.S.
Medical Student
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The transesophageal echocardiography images and videos show mild thickening of the anterior and posterior mitral valve leaflets, along with a highly mobile oscillating mass that can be visualized on the atrial aspect of the posterior mitral valve leaflet seen in Image A (red arrow) and video A. The mass is consistent with a vegetation, commonly diagnosed in patients with Infective Endocarditis (IE). Auscultation of a new murmur on cardiac physical examination, which reflects valvular damage due to the infection and subsequent valvular regurgitation, is a sign of endocarditis. This phenomenon is illustrated by the mild to moderate mitral valve regurgitation seen in Image B (video B).
IE has a relatively rare occurrence – a recent study found the 2011 incidence in the United States to be 15 per 100,000 persons, a slight increase from an incidence of 11 per 100,000 persons in 2000 [1]. The endothelium lining the valves of the healthy heart is designed to resist infective processes, but when the inner lining becomes damaged or a particularly virulent microorganism invades the bloodstream, infective endocarditis may occur, most commonly on the aortic or mitral valves. Endothelial disruption on the valvular surface may be caused by congenital heart defects, valvular dysfunction, the presence of a prosthetic valve, or prior infection, amongst other things. The damage of the endothelial cells leads to activation of the clotting cascade and the release of various inflammatory cytokines, eventually forming a thrombus, composed primarily of platelets and fibrin. The newly formed thrombus serves as a nidus for bacterial colonization during any period of transient bacteremia. This colonization results in the release of more inflammatory and clotting factors, creating a feed-forward type of mechanism that ultimately ends with the formation of a vegetation. Alternatively, infection can occur on a relatively normal valve in the setting of bacteremia with a more virulent organism, most commonly Staphylococcus aureus [2]. The diagnosis of endocarditis is largely guided by the use of the modified Duke criteria. Using this approach 2 major, 1 major and 3 minor, or 5 minor criteria are necessary to definitively make the diagnosis of infective endocarditis. The major criteria consist of specific parameters surrounding positive blood cultures as well as information supporting the involvement of the endocardium, most commonly provided through echocardiography, although other imaging modalities such as cardiac CT can be used [2,3]. Like any serious infection, endocarditis can be accompanied by numerous complications such as heart failure, abscess formation, and heart block, due to compromise of the cardiac conduction system in the setting of perivalvular extension [4]. Traditionally, IE is treated with parenteral antibiotics, although recent investigation has shown that transitioning to oral antibiotics in clinically stable patients may be acceptable [5]. Surgical intervention may be indicated in certain scenarios including a large vegetation (typically >10 mm) which carries a high risk for embolization, a resistant and progressive infection or, most commonly, in the event of severe valvular dysfunction [2,3]. The mortality rate associated with IE has shown minimal improvement throughout the years, remaining close to 30% in most cases. However, rates vary from case to case and differ based on the location, the infectious organism, the acuity, and associated comorbid conditions [3].
References:
- Pant S, Patel NJ, Deshmukh A, et al. Trends in Infective Endocarditis Incidence, Microbiology, and Valve Replacement in the United States From 2000 to 2011. Journal of the American College of Cardiology. 2015 May 19; 65(19): 2070–76.
- Cahill TJ, Prendergast BD. Infective Endocarditis. The Lancet. 2016 Mar 4; 387(10021): 882–93.
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. Journal of the American College of Cardiology. 2017 Jan 24; 69(3): 325–44.
- Graupner C, Vilacosta I, SanRomán JA, et al. Periannular Extension of Infective Endocarditis. Journal of the American College of Cardiology. 2002 Apr 3; 39(7): 1204–11.
- Iversen K, Ihlemann N, Gill SU, et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. New England Journal of Medicine. 2019 Jan 31; 380: 415-24.
Authors:
Morgan Roberts, B.S.
Medical Student
University of South Alabama
Mobile, AL
Galen Garriga, B.S.
Medical Student
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Ball & Cage Prosthetic Valve! An Obsolete Giant!
Description
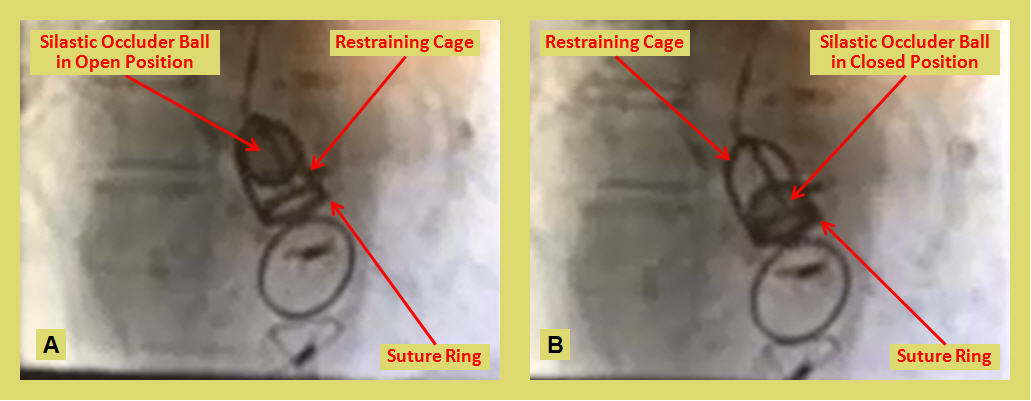
The cine still images and video show a ball and cage (Starr Edwards) aortic prosthetic valve in the open position in systole (A) and in the closed position in diastole (B). This type of prosthesis, once a giant in valve replacement surgery, is currently obsolete due to the high turbulent non-laminar flow, largely overcome by the more recent tilting disk valve prosthesis design.
The need for prosthetic heart valves was recognized long time ago, but the idea remained wishful and initially impractical. In 1912, Dr.Theodore Tuffier used his finger to free the fused leaflets of a stenosed aortic valve [1]. This was the first “closed heart procedure.” He was able to dilate the valve by pushing the invaginated aortic wall through the stenotic aortic valve.
In 1952 Dr. Charles Hufnagel implanted an acrylic ball encased in Lucite into the descending aorta of a 30 years old woman to correct aortic insufficiency at Georgetown Medical center, Washington D.C [2,3]. This was an initial step of a long journey more than half a century ago. Greater than 200 implanted Hufangel valves functioned for as long as 30 years with no significant wear; without use of anticoagulation. The downside of this design was that it could only be placed in the descending aorta instead of heart itself. It carried high mortality rate and the implantation procedure was cumbersome. Patients could hear the plastic ball bouncing inside their chest. Later, a hollow nylon ball coated with silicone rubber was used which reduced the valve noise. Although it had poor hemodynamic profile and could only give symptomatic relief, it proved that synthetic materials could be used to develop heart valves [4].
It was the development of the heart-lung machine in 1953 by Dr. John Gibbon at Thomas Jefferson hospital in Philadelphia that allowed cardiac surgery to blossom. In 1960, Dr. Harkins and his colleagues from Rhode Island started the modern era of prosthetic aortic valve replacement by inserting a double caged-ball valve into the aortic orifice below coronary ostia following the excision of diseased cusps [5]. In the mid1960s, Albert Starr (a physician) and Lowell Edwards (an electrical engineer) simplified the caged-ball valve by using a single titanium cage, a silastic ball and a sewing ring covered with Teflon. This Starr-Edwards valve was first implanted in the mitral position in 1960 and later in the aortic position. It had a sewing ring which was easy to suture to the aortic annulus [6]. The caged-ball valves, however, had a non-physiologic hemodynamic profile. The central ball occluder caused lateralization of forward flow with high turbulence; and the large sewing ring resulted in a restricted effective orifice area [7].
In 1969 tilting-disk mechanical prostheses were developed which had more physiologic central flow. The Björk-Shiley valve was the first tilting-disk prosthesis that was widely used. It was designed with a central disk held in place by two struts. The open valve had two orifices and resistance to blood flow was related to disk design and degree of opening angle. The disk design was progressively modified into a convexo-concave shape that could slide about 2 mm during its movement, increasing the effective orifice area [8]. Bjork-Shiley valves were recalled due to fracture of welded struts.
In 1977 bileaflet prostheses were developed by St. Jude Medical which consisted of two semicircular disks. This design produced three flow areas with a more uniform and laminar central flow. These valves provide greater effective orifice area and are least thrombogenic in comparison to other prosthetic valves [9,10]. In 1996, the On-X valve bileaflet tilting disk prosthesis was introduced with a more laminar flow design and less thrombogenicity, requiring lower anticoagulation targets [11].
References:
Authors:
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarah Hamid, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Grace Wenzel, M.D.
Staff Cardiologist
Penn Highlands Healthcare
DuBois, PA
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The cine still images and video show a ball and cage (Starr Edwards) aortic prosthetic valve in the open position in systole (A) and in the closed position in diastole (B). This type of prosthesis, once a giant in valve replacement surgery, is currently obsolete due to the high turbulent non-laminar flow, largely overcome by the more recent tilting disk valve prosthesis design.
The need for prosthetic heart valves was recognized long time ago, but the idea remained wishful and initially impractical. In 1912, Dr.Theodore Tuffier used his finger to free the fused leaflets of a stenosed aortic valve [1]. This was the first “closed heart procedure.” He was able to dilate the valve by pushing the invaginated aortic wall through the stenotic aortic valve.
In 1952 Dr. Charles Hufnagel implanted an acrylic ball encased in Lucite into the descending aorta of a 30 years old woman to correct aortic insufficiency at Georgetown Medical center, Washington D.C [2,3]. This was an initial step of a long journey more than half a century ago. Greater than 200 implanted Hufangel valves functioned for as long as 30 years with no significant wear; without use of anticoagulation. The downside of this design was that it could only be placed in the descending aorta instead of heart itself. It carried high mortality rate and the implantation procedure was cumbersome. Patients could hear the plastic ball bouncing inside their chest. Later, a hollow nylon ball coated with silicone rubber was used which reduced the valve noise. Although it had poor hemodynamic profile and could only give symptomatic relief, it proved that synthetic materials could be used to develop heart valves [4].
It was the development of the heart-lung machine in 1953 by Dr. John Gibbon at Thomas Jefferson hospital in Philadelphia that allowed cardiac surgery to blossom. In 1960, Dr. Harkins and his colleagues from Rhode Island started the modern era of prosthetic aortic valve replacement by inserting a double caged-ball valve into the aortic orifice below coronary ostia following the excision of diseased cusps [5]. In the mid1960s, Albert Starr (a physician) and Lowell Edwards (an electrical engineer) simplified the caged-ball valve by using a single titanium cage, a silastic ball and a sewing ring covered with Teflon. This Starr-Edwards valve was first implanted in the mitral position in 1960 and later in the aortic position. It had a sewing ring which was easy to suture to the aortic annulus [6]. The caged-ball valves, however, had a non-physiologic hemodynamic profile. The central ball occluder caused lateralization of forward flow with high turbulence; and the large sewing ring resulted in a restricted effective orifice area [7].
In 1969 tilting-disk mechanical prostheses were developed which had more physiologic central flow. The Björk-Shiley valve was the first tilting-disk prosthesis that was widely used. It was designed with a central disk held in place by two struts. The open valve had two orifices and resistance to blood flow was related to disk design and degree of opening angle. The disk design was progressively modified into a convexo-concave shape that could slide about 2 mm during its movement, increasing the effective orifice area [8]. Bjork-Shiley valves were recalled due to fracture of welded struts.
In 1977 bileaflet prostheses were developed by St. Jude Medical which consisted of two semicircular disks. This design produced three flow areas with a more uniform and laminar central flow. These valves provide greater effective orifice area and are least thrombogenic in comparison to other prosthetic valves [9,10]. In 1996, the On-X valve bileaflet tilting disk prosthesis was introduced with a more laminar flow design and less thrombogenicity, requiring lower anticoagulation targets [11].
References:
- Tuffier T. État actuel de la chirurgie intrathoracique. Trans Int Congr Med. 1913 7; Surgery 1914;2:249.
- Hufnagel CA. Aortic plastic valvular prosthesis. Bull Georgetown Univ Med Center. 1951;5:128–30.
- Hufnagel CA, Harvey WP, Rabil PJ, McDermott TF. Surgical correction of aortic insufficiency. Surgery 1954;35:673-83.
- Rajashekar, P. Development of mechanical heart valves - an inspiring tale: J of the practice of cardiovascular sciences. 2015;3:289-293
- Harken DE, Taylor WJ, Lefemine AA, Lunzer S, Low HB, Cohen ML, et al. Aortic valve replacement with a caged ball valve. Am J Cardiol 1962;9:292-9.
- Starr A, Edwards ML. Mitral replacement: Clinical experience with a ball-valve prosthesis. Ann Surg. 1961;154:726–40.
- Chaikof EL. The Development of Prosthetic Heart Valves – Lessons in Form and Function. N Engl J Med. 2007;357:1368–71.
- Lindblum D, Rodriguez L, Bjork VO. Mechanical failure of the Bjork-Shiley valve: updated follow-up and considerations on prophylactic replacement. J Thorac Cardiovasc Surg. 1989;97:95–7.
- Nicoloff DM, Emery RW, Arom KV, et al. Clinical and hemodynamic results with the St. Jude Medical cardiac valve prosthesis. J Thorac Cardiovasc Surg. 1982;82:674–83.
- Czer LS, Chaux A, Matloff JM, et al: Ten-year experience with the St Jude Medical valve for primary valve replacement. J Thorac Cardiovasc Surg. 1990;100:44; discussion 54.
- Yanagawa B, Levitsky S, Puskas JD; PROACT Investigators. Reduced anticoagulation is safe in high-risk patients with the On-X mechanical aortic valve. Curr Opin Cardiol. 2015 Mar;30(2):140-145.
Authors:
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarah Hamid, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Grace Wenzel, M.D.
Staff Cardiologist
Penn Highlands Healthcare
DuBois, PA
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL