December 2020 Issue
ISSN 2689-291X
ISSN 2689-291X
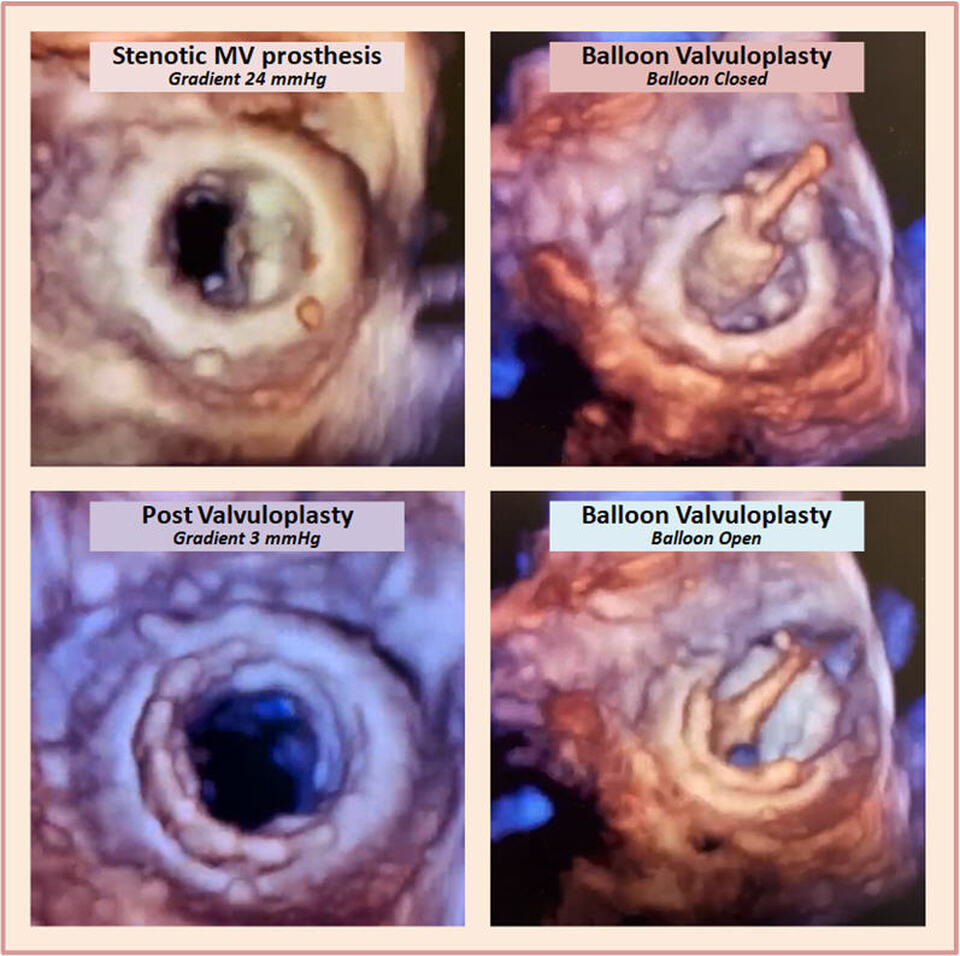
Mitral Prosthesis Balloon Valvuloplasty..3-D Style!
Description
The above 3-dimensional echocardiographic images (3-D) were acquired during balloon valvuloplasty of a stenotic mitral valve bioprosthesis. The gradual inflation of the balloon (see videos) resulted in successful increase in the valve area and significant decrease in the transvalvular gradient.
Discussion
Kanji Inoue, a Japanese cardiothoracic surgeon, introduced in 1982 the concept that a stenotic degenerative mitral valve can be dilated using a balloon [1]. This technique became widely used since then, with some procedural modification, and largely replaced surgical mitral commissurotomy in appropriate patient based on echocardiographic criteria [2].
Patient follow-up up to 20 years following percutaneous mitral valvuloplasty (PMV) reveals a good event-free survival; echocardiographic scoring based on available criteria of leaflet mobility, calcification, thickening and subvalvular thickening is vital prior to the procedure as it predicts long term events post PMV [3].
Two-dimensional echocardiography has emerged early on as a crucial tool in the assessment of stenotic mitral valves with regards to eligibility for PMV, and help in the selection of appropriate balloon size [4]. Echocardiographic guidance currently plays a crucial role in the success and safety of PMV [5].
Transesophageal echocardiography (TEE) provides added advantage and clarity allowing for greater detail and guidance during PMV [6]. More recently, the availability of real-time 3-dimensional (3-D) TEE has further improved the visualization of the mitral valve and commissures, providing better procedural guidance and assessment of procedural success [7]. Three-D TEE also helps in predicting the occurrence of post-PMV significant mitral valve regurgitation, a determinant of long term outcomes [8].
Intracardiac echocardiography (ICE) provides high-resolution real-time images of cardiac structures and early recognition of pericardial effusion with tamponade or intraluminal thrombi [9], and has recently gained grounds in PMV in addition to other percutaneous deployment of cardiac devices. It is well tolerated and can lead to less fluoroscopy time and radiation exposure.
Recent reports of PMV in the setting of a stenotic bioprosthetic mitral valve provide great insight on its feasibility and safety, allowing the delay or avoidance of surgical intervention [10].
References:
Authors:
Nikky Bardia, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Siva Chiranjeevi, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Nilarun Chowdhuri, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Usman Sarwar, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Maulikkumar Patel, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Amod Amritphale, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Abimbola Shofu, M.D.
Staff Cardiologist
Genesis Healthcare System
Zanesville, OH
The above 3-dimensional echocardiographic images (3-D) were acquired during balloon valvuloplasty of a stenotic mitral valve bioprosthesis. The gradual inflation of the balloon (see videos) resulted in successful increase in the valve area and significant decrease in the transvalvular gradient.
Discussion
Kanji Inoue, a Japanese cardiothoracic surgeon, introduced in 1982 the concept that a stenotic degenerative mitral valve can be dilated using a balloon [1]. This technique became widely used since then, with some procedural modification, and largely replaced surgical mitral commissurotomy in appropriate patient based on echocardiographic criteria [2].
Patient follow-up up to 20 years following percutaneous mitral valvuloplasty (PMV) reveals a good event-free survival; echocardiographic scoring based on available criteria of leaflet mobility, calcification, thickening and subvalvular thickening is vital prior to the procedure as it predicts long term events post PMV [3].
Two-dimensional echocardiography has emerged early on as a crucial tool in the assessment of stenotic mitral valves with regards to eligibility for PMV, and help in the selection of appropriate balloon size [4]. Echocardiographic guidance currently plays a crucial role in the success and safety of PMV [5].
Transesophageal echocardiography (TEE) provides added advantage and clarity allowing for greater detail and guidance during PMV [6]. More recently, the availability of real-time 3-dimensional (3-D) TEE has further improved the visualization of the mitral valve and commissures, providing better procedural guidance and assessment of procedural success [7]. Three-D TEE also helps in predicting the occurrence of post-PMV significant mitral valve regurgitation, a determinant of long term outcomes [8].
Intracardiac echocardiography (ICE) provides high-resolution real-time images of cardiac structures and early recognition of pericardial effusion with tamponade or intraluminal thrombi [9], and has recently gained grounds in PMV in addition to other percutaneous deployment of cardiac devices. It is well tolerated and can lead to less fluoroscopy time and radiation exposure.
Recent reports of PMV in the setting of a stenotic bioprosthetic mitral valve provide great insight on its feasibility and safety, allowing the delay or avoidance of surgical intervention [10].
References:
- Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984 Mar;87(3):394-402.
- Nobuyoshi M, Arita T, Shirai S, Hamasaki N, Yokoi H, Iwabuchi M, Yasumoto H, Nosaka H. Percutaneous balloon mitral valvuloplasty: a review. Circulation. 2009 Mar 3;119(8):e211-9.
- Rodrigues I, Branco L, Patrício L, Bernardes L, Abreu J, Cacela D, Galrinho A, Ferreira R. Long-Term Follow Up After Successful Percutaneous Balloon Mitral Valvuloplasty. J Heart Valve Dis. 2017 Nov;26(6):659-666.
- Chen CG, Wang X, Wang Y, Lan YF. Value of two-dimensional echocardiography in selecting patients and balloon sizes for percutaneous balloon mitral valvuloplasty. J Am Coll Cardiol. 1989 Dec;14(7):1651-8.
- Liu Y, Guo GL, Wen B, Wang S, Ou-Yang WB, Xie Y, Pan XB. Feasibility and effectiveness of percutaneous balloon mitral valvuloplasty under echocardiographic guidance only. Echocardiography. 2018 Oct;35(10):1507-1511.
- Chan K, Marquis J, Ascah C, Morton B, Baird M. Role of transesophageal echocardiography in percutaneous balloon mitral valvuloplasty. Echocardiography. 1990 Mar;7(2):115-23.
- Eng MH, Salcedo EE, Kim M, Quaife RA, Carroll JD. Implementation of real-time three-dimensional transesophageal echocardiography for mitral balloon valvuloplasty. Catheter Cardiovasc Interv. 2013 Nov 15;82(6):994-8.
- Alkhouly AA, Al-Amin AM, Mukarrab MI. Role of three dimensional transesophageal echocardiography in predicting mitral regurgitation after percutaneous balloon mitral valvuloplasty. Indian Heart J. 2018 Nov-Dec;70(6):836-842.
- Ahmari SA, Amro A, Otabi MA, Abdullah MA, Kasab SA, Amri HA. Initial experience of using intracardiac echocardiography (ICE) for guiding balloon mitral valvuloplasty (BMV). J Saudi Heart Assoc. 2012 Jan;24(1):23-7.
- Hamatani Y, Saito N, Tazaki J, Natsuaki M, Nakai K, Makiyama T, Sasaki Y, Imai M, Watanabe S, Shioi T, Kimura T, Inoue K. Percutaneous balloon valvuloplasty for bioprosthetic mitral valve stenosis. Heart Vessels. 2013 Sep;28(5):667-71.
Authors:
Nikky Bardia, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Siva Chiranjeevi, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Nilarun Chowdhuri, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Usman Sarwar, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Maulikkumar Patel, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Amod Amritphale, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Abimbola Shofu, M.D.
Staff Cardiologist
Genesis Healthcare System
Zanesville, OH