February 2019 Issue
ISSN 2689-291X
ISSN 2689-291X
Challenging Images
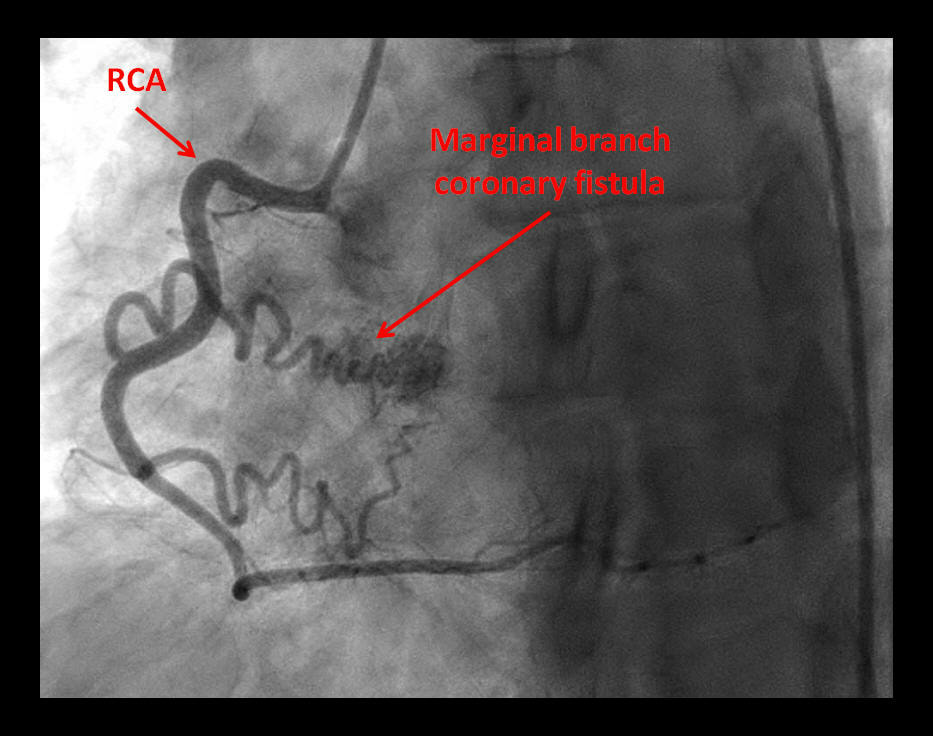
Coronary Fistula: The Anomalous Tributary!
Description
Coronary artery fistulae have been described draining coronary artery flow into another vascular structure or cardiac cavity [1]. These are mostly congenital [2], with infrequent cases of iatrogenic coronary fistulae reported [3]. Various management options have been proposed based on size and physiology of the fistula, complications and symptoms [4].
References
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Coronary artery fistulae have been described draining coronary artery flow into another vascular structure or cardiac cavity [1]. These are mostly congenital [2], with infrequent cases of iatrogenic coronary fistulae reported [3]. Various management options have been proposed based on size and physiology of the fistula, complications and symptoms [4].
References
- Raju MG, Goyal SK, Punnam SR, Shah DO, Smith GF, Abela GS. Coronary artery fistula: a case series with review of the literature. J Cardiol. 2009 Jun;53(3):467-72.
- Tkebuchava T, Von Segesser LK, Vogt PR, Jenni R, Arbenz U, Turina M. Congenital coronary fistulas in children and adults: diagnosis, surgical technique and results. J Cardiovasc Surg (Torino). 1996 Feb;37(1):29-34.
- Banerjee S, Patra S. Coronary-Cameral Fistula Caused by Guidewire Trauma and Resolved by Coil Embolization. Tex Heart Inst J. 2016 Aug 1;43(4):338-40.
- Ata Y, Turk T, Bicer M, Yalcin M, Ata F, Yavuz S. Coronary arteriovenous fistulas in the adults: natural history and management strategies. J Cardiothorac Surg. 2009 Nov 6;4:62.
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Journal Review
Sacubitril/Valsartan: A Pioneer in Acute Heart Failure
and a Paradigm in Chronic Heart Failure
Abstract
Sacubitril/valsartan (neprilysin inhibitor/angiotensin receptor blocker combination) was compared to enalapril in stable patients who had heart failure with reduced ejection fraction (HFrEF) in the PARADIGM-HF trial [1]. In the 8442 patients with class II, III, or IV heart failure and an ejection fraction of 40% or less, this combination resulted in the primary outcome (a composite of death from cardiovascular causes or hospitalization for heart failure) in 21.8% of patients compared to 26.5% of patients in the enalapril group. As compared with enalapril, this combination also reduced the risk of hospitalization for heart failure by 21% (P<0.001) and decreased the symptoms and physical limitations of heart failure (P=0.001). The main risks of this therapy were hypotension and angioedema.
The current issue of the New England Journal of Medicine [2] reported the findings of the PIONEER-HF clinical trial, which examined the role of angiotensin–neprilysin inhibition in acute decompensated heart failure.
Methods
Results
Conclusion
Among patients with heart failure with reduced ejection fraction (HFrEF) who were hospitalized for acute decompensated heart failure, the initiation of sacubitril–valsartan therapy led to a greater reduction in the NT-proBNP concentration than enalapril therapy.
Rates of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema did not differ significantly between the two groups.
Clinical Implications
Heart failure remains a very common and lethal disease, associated with enormous cost, mostly from hospital admissions, despite recent novel pharmacologic and nonpharmacologic advances in its treatment [3]. The concept of neprilysin inhibition in combination with angiotensin receptor inhibition is novel, and appears to have gained an important niche in the guideline-directed treatment of heart failure with reduced ejection fraction [4]. Nevertheless, it remains very important to address medication adherence in the treatment of heart failure [5] especially in light of the soaring costs of heart failure medications [6] and the polypharmacy expected to achieve better outcomes [7]. Patient-centered therapy, examining “what works best” taking into consideration these factors may ultimately provide the desired outcomes [8].
References
Authors:
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Sacubitril/valsartan (neprilysin inhibitor/angiotensin receptor blocker combination) was compared to enalapril in stable patients who had heart failure with reduced ejection fraction (HFrEF) in the PARADIGM-HF trial [1]. In the 8442 patients with class II, III, or IV heart failure and an ejection fraction of 40% or less, this combination resulted in the primary outcome (a composite of death from cardiovascular causes or hospitalization for heart failure) in 21.8% of patients compared to 26.5% of patients in the enalapril group. As compared with enalapril, this combination also reduced the risk of hospitalization for heart failure by 21% (P<0.001) and decreased the symptoms and physical limitations of heart failure (P=0.001). The main risks of this therapy were hypotension and angioedema.
The current issue of the New England Journal of Medicine [2] reported the findings of the PIONEER-HF clinical trial, which examined the role of angiotensin–neprilysin inhibition in acute decompensated heart failure.
Methods
- HfrEF patients who were hospitalized for acute decompensated heart failure were included.
- After hemodynamic stabilization, patients were randomly assigned to receive sacubitril–valsartan (97 mg of sacubitril with 103 mg of valsartan twice daily) or enalapril (10 mg twice daily).
- The primary efficacy outcome was the time-averaged proportional change in the N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentration from baseline through weeks 4 and 8.
- Key safety outcomes were the rates of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema.
Results
- 881 patients were randomized:
- 440 sacubitril–valsartan
- 441 enalapril.
- Time-averaged reduction in the NT-proBNP concentration was significantly greater in the sacubitril–valsartan group than in the enalapril group
- The greater reduction in the NT-proBNP concentration with sacubitril–valsartan than with enalapril was evident as early as week 1.
- Rates of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema did not differ significantly between the two groups.
Conclusion
Among patients with heart failure with reduced ejection fraction (HFrEF) who were hospitalized for acute decompensated heart failure, the initiation of sacubitril–valsartan therapy led to a greater reduction in the NT-proBNP concentration than enalapril therapy.
Rates of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema did not differ significantly between the two groups.
Clinical Implications
Heart failure remains a very common and lethal disease, associated with enormous cost, mostly from hospital admissions, despite recent novel pharmacologic and nonpharmacologic advances in its treatment [3]. The concept of neprilysin inhibition in combination with angiotensin receptor inhibition is novel, and appears to have gained an important niche in the guideline-directed treatment of heart failure with reduced ejection fraction [4]. Nevertheless, it remains very important to address medication adherence in the treatment of heart failure [5] especially in light of the soaring costs of heart failure medications [6] and the polypharmacy expected to achieve better outcomes [7]. Patient-centered therapy, examining “what works best” taking into consideration these factors may ultimately provide the desired outcomes [8].
References
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014 Sep 11;371(11):993-1004.2.
- Velazquez EJ, Morrow DA, DeVore AD, et al. PIONEER-HF Investigators.Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019 Feb 7;380(6):539-548.3.
- Callan PD, Clark AL. Heart failure - what's new and what's changed? Clin Med (Lond). 2016 Dec;16(Suppl 6):s37-s42.4.
- Rodgers JE. Sacubitril/Valsartan: The Newest Addition to the Toolbox for Guideline-Directed Medical Therapy of Heart Failure. Am J Med. 2017 Jun;130(6):635-639.5.
- Ruppar TM, Cooper PS, Mehr DR, et al. Medication Adherence Interventions Improve Heart Failure Mortality and Readmission Rates: Systematic Review and Meta-Analysis of Controlled Trials. J Am Heart Assoc. 2016 Jun 17;5(6).6.
- Hussey LC, Hardin S, Blanchette C. Outpatient costs of medications for patients with chronic heart failure. Am J Crit Care. 2002 Sep;11(5):474-8.7.
- Mastromarino V, Casenghi M, Testa M, et al. Polypharmacy in heart failure patients. Curr Heart Fail Rep. 2014 Jun;11(2):212-9.
- Ekman I, Wolf A, Olsson LE, et al. Effects of person-centred care in patients with chronic heart failure: the PCC-HF study. Eur Heart J. 2012 May;33(9):1112-9.
Authors:
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Journal Review
Keeping Up with the Trend: Finally a Randomized
Clinical Trial for E – Cigarettes!
Abstract
E – Cigarettes have been popular with the public since their emergence in the mid 2000s. Often, patients think that e – cigarettes do not carry any major risk and are good alternatives to tobacco products. However, their efficacy in aiding smoking cessation is unknown. A randomized trial from the United Kingdom seeks to compare e – cigarettes to current smoking-cessation treatments (1).
Methods
866 participants were randomized to either nicotine – replacement products of their choice (combinations are accepted) or an e – cigarette starter pack, for up to 3 months. Nicotine replacement products include patch, gum, lozenge, nasal spray, inhalator, mouth spray, mouth strip, and microtabs. The e-cigarette starter pack is called One Kit, and contains 18 mg/mL nicotine concentration. Weekly behavioral support for a minimum of 4 weeks is included in both groups. Primary outcome is abstinence at one year. Secondary outcomes are self-reported treatment usage and respiratory symptoms. Product distribution is not blinded.
Results
Discussion
In this study shown by Hajek et al, e-cigarette is more successful at smoking cessation than nicotine – replacement products. However, there are still many important questions about e-cigarettes that are unanswered. At one year, the e-cigarette group had a higher percentage of continuation of the product. The long term effects e-cigarettes have on health are still unknown at this time (2). In addition, both e-cigarettes and nicotine-replacement groups also utilized behavioral therapy as aid, increasing their success rate (3). Therefore, at this time, e-cigarette usage as smoking cessation aid must be considered carefully, rather than being viewed as a risk-free alternative to cigarettes, to avoid potential widespread harm (4).
References
Authors:
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
E – Cigarettes have been popular with the public since their emergence in the mid 2000s. Often, patients think that e – cigarettes do not carry any major risk and are good alternatives to tobacco products. However, their efficacy in aiding smoking cessation is unknown. A randomized trial from the United Kingdom seeks to compare e – cigarettes to current smoking-cessation treatments (1).
Methods
866 participants were randomized to either nicotine – replacement products of their choice (combinations are accepted) or an e – cigarette starter pack, for up to 3 months. Nicotine replacement products include patch, gum, lozenge, nasal spray, inhalator, mouth spray, mouth strip, and microtabs. The e-cigarette starter pack is called One Kit, and contains 18 mg/mL nicotine concentration. Weekly behavioral support for a minimum of 4 weeks is included in both groups. Primary outcome is abstinence at one year. Secondary outcomes are self-reported treatment usage and respiratory symptoms. Product distribution is not blinded.
Results
- The abstinence rate was 18% in the e – cigarette group, and 9.9% in the nicotine – replacement group (P < 0.001).
- At 52 weeks, 80% of the e-cigarette group was still using their products, compared to 9% of the nicotine-replacement group.
- Throat or mouth irritation were reported more in the e-cigarette group (65.3%) compared to nicotine-replacement group (51.2%).
- Nausea was reported more frequently in the nicotine-replacement group (37.9%) versus the e-cigarette group (31.3%).
- No significant difference in incidence of wheezing or shortness of breath between both groups
Discussion
In this study shown by Hajek et al, e-cigarette is more successful at smoking cessation than nicotine – replacement products. However, there are still many important questions about e-cigarettes that are unanswered. At one year, the e-cigarette group had a higher percentage of continuation of the product. The long term effects e-cigarettes have on health are still unknown at this time (2). In addition, both e-cigarettes and nicotine-replacement groups also utilized behavioral therapy as aid, increasing their success rate (3). Therefore, at this time, e-cigarette usage as smoking cessation aid must be considered carefully, rather than being viewed as a risk-free alternative to cigarettes, to avoid potential widespread harm (4).
References
- Hajek P, Phillips-Waller A, Przulj D, et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med. 2019 Feb 14;380(7):629-637.
- Bals R, Boyd J, Esposito S, et al. Electronic cigarettes: a task force report from the European Respiratory Society. Eur Respir J. 2019 Jan 31;53(2). pii: 1801151.
- Lunden SE, Pittman JC, Prashad N, et al. Cognitive, Behavioral, and Situational Influences on Relapse to Smoking After Group Treatment for Tobacco Dependence. Front Psychol. 2019 Jan 30;9:2756.
- Livingston CJ, Freeman RJ, Costales VC, et al. Electronic Nicotine Delivery Systems or E-cigarettes: American College of Preventive Medicine's Practice Statement. Am J Prev Med. 2019 Jan;56(1):167-178.
Authors:
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL