February 2021 Issue
ISSN 2689-291X
ISSN 2689-291X
Infiltrative Cardiomyopathy..
ECG/Echo Hypertrophy Paradox!
Description
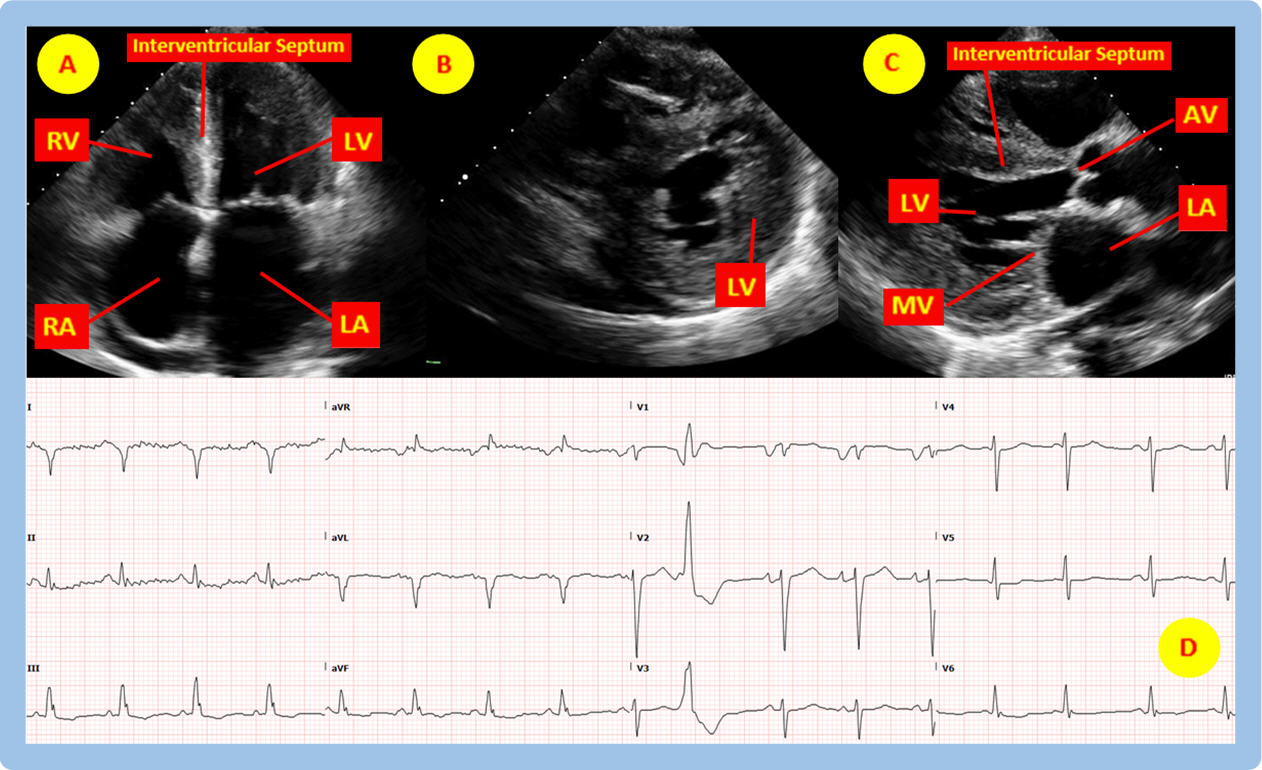
Figure A above (see accompanying video) is a 2-dimensional (2-D) transthoracic echocardiography (TTE) image in the apical four chamber (A4C) view showing marked hypertrophy of the interventricular septum and the lateral wall of the left ventricle (LV), hypertrophy of the right ventricular (RV) free wall, in addition to left atrial (LA) and right atrial (RA) dilation, all feature of hypertrophic versus infiltrative cardiomyopathy. Figure B in the TTE parasternal short axis (PSA) view demonstrates concentric thickening of all segments of the LV. Figure C is a parasternal long axis (PLA) view depicting a decreased LV cavity size resulting from the concentric LV hypertrophy. Figure D shows the corresponding electrocardiogram (ECG) without evidence of hypertrophy by voltage and with paradoxical relatively low voltage in the limb leads despite the left ventricular hypertrophy on TTE, all suggestive of infiltrative disease as the cause of the hypertrophy seen on TTE. The poor R wave progression in the precordial leads and the presence of Q waves in the high lateral leads in the absence of wall motion abnormalities on the TTE also lend supportive evidence to infiltrative cardiomyopathy.
Discussion
Restrictive cardiomyopathy is defined as increased myocardial stiffening that eventually results in diastolic dysfunction with preserved ejection fraction. Etiologies of restrictive cardiomyopathy range from infiltrative diseases such as amyloidosis and glycogen/lysosomal storage diseases to non-infiltrative diseases such as fibrosis of the endocardium [1]. Restrictive cardiomyopathy should be suspected in patients who have low to normal QRS voltages in all leads on ECG while there is left ventricular hypertrophy apparent on an echocardiogram [2]. Typically, left ventricular hypertrophy in hypertrophic cardiomyopathy results in increased QRS voltage amplitudes on ECG due to increased muscle mass. However, with most restrictive cardiomyopathies due to infiltrative disease, the increase in ventricular wall thickness is not due to myocyte hypertrophy of the myocardium but rather due to infiltration of the myocardium with non-contractile compounds. The voltage/mass ratio is a helpful tool to identify restrictive/infiltrative myocardial physiology due to its prediction of the inverse relationship between left ventricular QRS voltage on ECG and mass on TTE [2].
The above figures are indicative of infiltrative cardiomyopathy, most commonly cardiac amyloidosis. The left and right ventricles have thickened walls with small chamber sizes and with bi-atrial enlargement due to impaired myocardial filling resulting in elevated atrial filling pressures. Thickening of the ventricles can result in abnormal geometry of the atrioventricular valves (tricuspid and mitral valves) which can cause varying degrees of mitral and tricuspid regurgitation (visible on color Doppler in the accompanying video). As discussed above, the ECG paradoxically demonstrates no voltage criteria for hypertrophy, in addition to relatively low QRS voltages (5-6 mm in height) especially in the limb leads, despite the left ventricular hypertrophy on the echocardiogram [3]. Signs and symptoms of restrictive cardiomyopathy are similar to typical signs of heart failure including jugular venous distension (JVD), lower extremity edema, dyspnea, and eventually signs of low cardiac output such as syncope and diminished capillary refill [4].
There are two main causes of cardiac amyloidosis: AL amyloidosis and transthyretin amyloidosis (ATTR) [4]. The general pathogenesis of cardiac amyloidosis is the deposition and aggregation of misfolded insoluble proteins in cardiac tissue. In AL amyloidosis the misfolded proteins are derived from immunoglobulin light chains (associated with multiple myeloma) and in ATTR the transthyretin protein is a protein synthesized in the liver (also known as prealbumin). Confirmatory imaging includes cardiac magnetic resonance and bone scintigraphy [3]. Under polarized light microscopy, biopsy samples with Congo red dye will demonstrate apple green birefringence.
There are two objectives in treating cardiac amyloidosis. First is to treat the heart failure symptoms; next is to treat the underlying disease [5]. Loop diuretics are used with caution due to the potential of over-diuresis which could result in significant hypotension. ACE inhibitors and beta blockers, although have a morbidity/mortality benefit in dilated cardiomyopathy with systolic heart failure, are often not tolerated well in patients with restrictive physiology due to a decrease in preload and afterload which can result in profound hypotension [5, 6]. Treating the underlying disease in AL amyloidosis involves treating the multiple myeloma with chemotherapeutic agents such as dexamethasone, cyclophosphamide, and bortezomib [5]. Treating the underlying disease of ATTR involves liver transplant due to the synthesis of transthyretin in the liver [6]. Pharmacologic agents such as tafamidis, act to stabilize transthyretin and prevent the formation of amyloid, and have shown some clinical promise [6].
References:
Authors:
Hadil El-Sharkh, B.S.
Medical Student (MIII)
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Figure A above (see accompanying video) is a 2-dimensional (2-D) transthoracic echocardiography (TTE) image in the apical four chamber (A4C) view showing marked hypertrophy of the interventricular septum and the lateral wall of the left ventricle (LV), hypertrophy of the right ventricular (RV) free wall, in addition to left atrial (LA) and right atrial (RA) dilation, all feature of hypertrophic versus infiltrative cardiomyopathy. Figure B in the TTE parasternal short axis (PSA) view demonstrates concentric thickening of all segments of the LV. Figure C is a parasternal long axis (PLA) view depicting a decreased LV cavity size resulting from the concentric LV hypertrophy. Figure D shows the corresponding electrocardiogram (ECG) without evidence of hypertrophy by voltage and with paradoxical relatively low voltage in the limb leads despite the left ventricular hypertrophy on TTE, all suggestive of infiltrative disease as the cause of the hypertrophy seen on TTE. The poor R wave progression in the precordial leads and the presence of Q waves in the high lateral leads in the absence of wall motion abnormalities on the TTE also lend supportive evidence to infiltrative cardiomyopathy.
Discussion
Restrictive cardiomyopathy is defined as increased myocardial stiffening that eventually results in diastolic dysfunction with preserved ejection fraction. Etiologies of restrictive cardiomyopathy range from infiltrative diseases such as amyloidosis and glycogen/lysosomal storage diseases to non-infiltrative diseases such as fibrosis of the endocardium [1]. Restrictive cardiomyopathy should be suspected in patients who have low to normal QRS voltages in all leads on ECG while there is left ventricular hypertrophy apparent on an echocardiogram [2]. Typically, left ventricular hypertrophy in hypertrophic cardiomyopathy results in increased QRS voltage amplitudes on ECG due to increased muscle mass. However, with most restrictive cardiomyopathies due to infiltrative disease, the increase in ventricular wall thickness is not due to myocyte hypertrophy of the myocardium but rather due to infiltration of the myocardium with non-contractile compounds. The voltage/mass ratio is a helpful tool to identify restrictive/infiltrative myocardial physiology due to its prediction of the inverse relationship between left ventricular QRS voltage on ECG and mass on TTE [2].
The above figures are indicative of infiltrative cardiomyopathy, most commonly cardiac amyloidosis. The left and right ventricles have thickened walls with small chamber sizes and with bi-atrial enlargement due to impaired myocardial filling resulting in elevated atrial filling pressures. Thickening of the ventricles can result in abnormal geometry of the atrioventricular valves (tricuspid and mitral valves) which can cause varying degrees of mitral and tricuspid regurgitation (visible on color Doppler in the accompanying video). As discussed above, the ECG paradoxically demonstrates no voltage criteria for hypertrophy, in addition to relatively low QRS voltages (5-6 mm in height) especially in the limb leads, despite the left ventricular hypertrophy on the echocardiogram [3]. Signs and symptoms of restrictive cardiomyopathy are similar to typical signs of heart failure including jugular venous distension (JVD), lower extremity edema, dyspnea, and eventually signs of low cardiac output such as syncope and diminished capillary refill [4].
There are two main causes of cardiac amyloidosis: AL amyloidosis and transthyretin amyloidosis (ATTR) [4]. The general pathogenesis of cardiac amyloidosis is the deposition and aggregation of misfolded insoluble proteins in cardiac tissue. In AL amyloidosis the misfolded proteins are derived from immunoglobulin light chains (associated with multiple myeloma) and in ATTR the transthyretin protein is a protein synthesized in the liver (also known as prealbumin). Confirmatory imaging includes cardiac magnetic resonance and bone scintigraphy [3]. Under polarized light microscopy, biopsy samples with Congo red dye will demonstrate apple green birefringence.
There are two objectives in treating cardiac amyloidosis. First is to treat the heart failure symptoms; next is to treat the underlying disease [5]. Loop diuretics are used with caution due to the potential of over-diuresis which could result in significant hypotension. ACE inhibitors and beta blockers, although have a morbidity/mortality benefit in dilated cardiomyopathy with systolic heart failure, are often not tolerated well in patients with restrictive physiology due to a decrease in preload and afterload which can result in profound hypotension [5, 6]. Treating the underlying disease in AL amyloidosis involves treating the multiple myeloma with chemotherapeutic agents such as dexamethasone, cyclophosphamide, and bortezomib [5]. Treating the underlying disease of ATTR involves liver transplant due to the synthesis of transthyretin in the liver [6]. Pharmacologic agents such as tafamidis, act to stabilize transthyretin and prevent the formation of amyloid, and have shown some clinical promise [6].
References:
- N. L. Pereira, M. Grogan, and G. W. Dec, “Spectrum of Restrictive and Infiltrative Cardiomyopathies,” J. Am. Coll. Cardiol., vol. 71, no. 10, pp. 1130–1148, Mar. 2018.
- R. H. Falk and C. C. Quarta, “Echocardiography in cardiac amyloidosis,” Heart Fail. Rev., vol. 20, no. 2, pp. 125–131, Mar. 2015.
- Martinez-Naharro, P. N. Hawkins, and M. Fontana, “Cardiac amyloidosis,” Clin. Med. Lond. Engl., vol. 18, no. Suppl 2, pp. s30–s35, Apr. 2018.
- M. Fontana, A. Ćorović, P. Scully, and J. C. Moon, “Myocardial Amyloidosis,” JACC Cardiovasc. Imaging, vol. 12, no. 11, pp. 2345–2356, Nov. 2019.
- R. H. Falk, K. M. Alexander, R. Liao, and S. Dorbala, “AL (Light-Chain) Cardiac Amyloidosis,” J. Am. Coll. Cardiol., vol. 68, no. 12, pp. 1323–1341, Sep. 2016.
- M. A. Gertz et al., “Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis,” J. Am. Coll. Cardiol., vol. 66, no. 21, pp. 2451–2466, Dec. 2015.
Authors:
Hadil El-Sharkh, B.S.
Medical Student (MIII)
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL