January 2020 Issue
ISSN 2689-291X
ISSN 2689-291X
Challenging Images
Renal Cell Carcinoma..Cardiac Extension & Mets!
Description
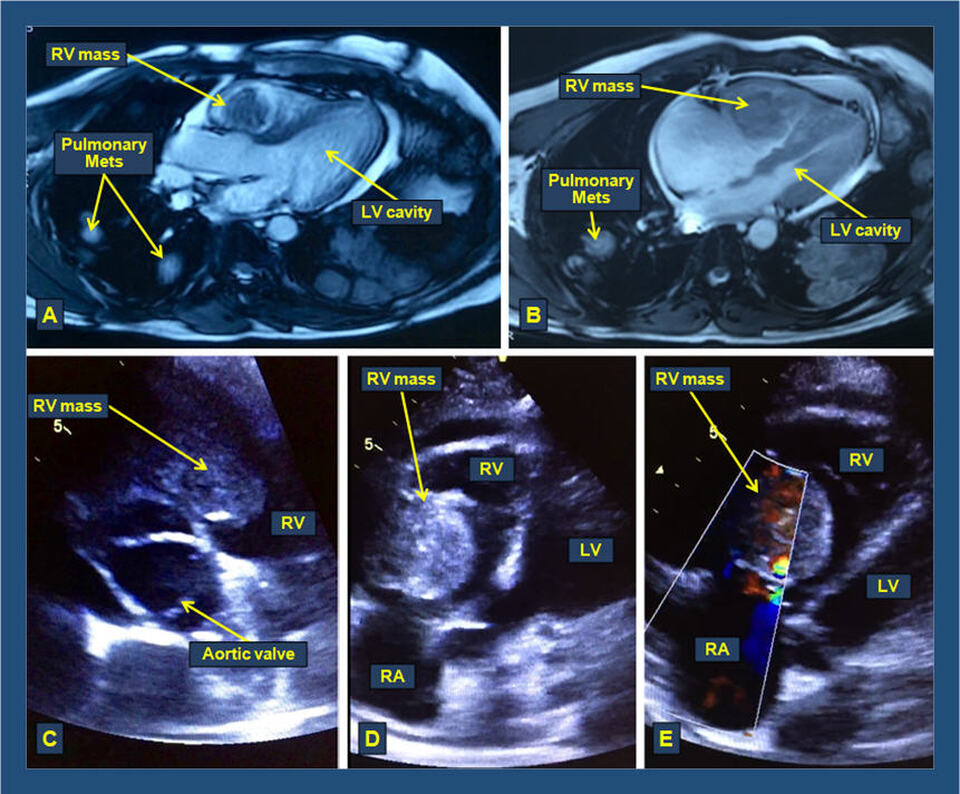
The figure above shows computed tomography (CT) axial images of the chest (A & B) with a right ventricular mass, representing extension of renal cell carcinoma (Grawitz tumor) through the inferior vena cava into the right atrium (RA) and the right ventricle (RV). Pulmonary nodules representing pulmonary metastases can also be seen. The 2-dimensional echocardiography (2-D Echo) views reveal the mass in the in the parasternal short access view at the level of the aortic valve (C). The mass is also clearly seen along the RV base (D) in the modified subcostal view (D), with color flow Doppler imaging (E) revealing vascularity of the mass and trivial tricuspid regurgitation.
Discussion
Renal cell carcinoma (RCC), which comprises about 2% of all cancers, is metastatic in 30 % of the cases upon diagnosis due to the lack of early warning signs and variable manifestations [1]. Intramyocardial metastasis of the left ventricle and interventricular septum have been reported with adequate response to pazopanib [2]. Heart failure due to nonischemic cardiomyopathy has also been reported as the initial presenting symptoms of RCC, which improved following tumor resection [3]. Heart failure as a paraneoplastic manifestation has been reported, which was reversed upon therapy with pazopanib [4]. Right-sided heart failure was also reported as a manifestation of metastatic RCC mimicking obstruction [5]. Although commonly thought of as an invasive tumor, traveling up the inferio vena cava to the right atrium, cases of cardiac mets without inferior vena cava or right atrial involvement have been reported [6]. Rarely, RCC can metastasize to the left heart, including the left atrium and coronary sinus [7], in addition to the left ventricle [8]. The primary treatment of RCC remains surgical, often in combination with chemotherapy, radiation therapy, and targeted immune therapy [9]. Classifying RCC into different risk groups helps predict outcomes and may allow better patient counseling and identify need for alternative treatment [10].
References:
Authors:
Mahmoud N. Hamad
Medical Student
Royal College of Surgeons
Dublin, Ireland
Sagar Kumar, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The figure above shows computed tomography (CT) axial images of the chest (A & B) with a right ventricular mass, representing extension of renal cell carcinoma (Grawitz tumor) through the inferior vena cava into the right atrium (RA) and the right ventricle (RV). Pulmonary nodules representing pulmonary metastases can also be seen. The 2-dimensional echocardiography (2-D Echo) views reveal the mass in the in the parasternal short access view at the level of the aortic valve (C). The mass is also clearly seen along the RV base (D) in the modified subcostal view (D), with color flow Doppler imaging (E) revealing vascularity of the mass and trivial tricuspid regurgitation.
Discussion
Renal cell carcinoma (RCC), which comprises about 2% of all cancers, is metastatic in 30 % of the cases upon diagnosis due to the lack of early warning signs and variable manifestations [1]. Intramyocardial metastasis of the left ventricle and interventricular septum have been reported with adequate response to pazopanib [2]. Heart failure due to nonischemic cardiomyopathy has also been reported as the initial presenting symptoms of RCC, which improved following tumor resection [3]. Heart failure as a paraneoplastic manifestation has been reported, which was reversed upon therapy with pazopanib [4]. Right-sided heart failure was also reported as a manifestation of metastatic RCC mimicking obstruction [5]. Although commonly thought of as an invasive tumor, traveling up the inferio vena cava to the right atrium, cases of cardiac mets without inferior vena cava or right atrial involvement have been reported [6]. Rarely, RCC can metastasize to the left heart, including the left atrium and coronary sinus [7], in addition to the left ventricle [8]. The primary treatment of RCC remains surgical, often in combination with chemotherapy, radiation therapy, and targeted immune therapy [9]. Classifying RCC into different risk groups helps predict outcomes and may allow better patient counseling and identify need for alternative treatment [10].
References:
- Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996 Sep 19;335(12):865-75.
- Czarnecka AM, Sobczuk P, Lian F, et al. Renal cell carcinoma with intramyocardial metastases. BMC Urol. 2014 Sep 6;14:73.
- Lee CM, Sim A, Kurugulasigamoney G, et al. Heart failure as the first manifestation of renal cell carcinoma. Korean J Urol. 2015 Jan;56(1):82-5.
- Pandey M, Gandhi S, George S. Heart Failure: A Paraneoplastic Manifestation of Renal Cell Carcinoma - Reversed With Pazopanib. Clin Genitourin Cancer. 2017 Oct;15(5):e835-e837.
- Sawant AC, Atla PR, Srivatsa SS, et al. Right heart failure caused by metastatic renal cell carcinoma masquerading as an obstructive cardiac tumour. BMJ Case Rep. 2012 Aug 8;2012.
- Sahin S, Karatas F, Hacioglu MB, et al. Renal cell carcinoma presenting with heart metastasis without inferior vena caval and right atrial involvement. J Cancer Res Ther. 2018 Jan-Mar;14(2):457-458.
- Nkengurutse G, Wang Q, Tian F, et al. Renal Cell Carcinoma Metastasizing to Left Atrium With Coronary Sinus Invasion: A Rare Site of Metastasis Mimicking Myxoma. Front Oncol. 2019 Aug 7;9:738.
- Aburto J, Bruckner BA, Blackmon SH, et al. Renal cell carcinoma, metastatic to the left ventricle. Tex Heart Inst J. 2009;36(1):48-9.
- Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin. 2017 Nov;67(6):507-524.
- Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol 2002; 20:4559–66.
Authors:
Mahmoud N. Hamad
Medical Student
Royal College of Surgeons
Dublin, Ireland
Sagar Kumar, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Trial Review
The COLCOT Trial: Colchicine For Secondary Post–MI Prevention!
Abstract
Inflammation has long been known to raise the risk of plaque rupture, leading to acute coronary syndromes [1]. Multiple trials have been done in the past to show the benefits of inflammation reduction [2]. The CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) trial showed that canakinumab can lower cardiovascular events when compared to placebo, due to inhibition of interleukin 1B [3]. The LoDoCo (Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease) trial demonstrated that patients receiving 0.5 mg daily of colchicine had fewer cardiovascular events than patients who did not receive colchicine [4]. Thus, with heart disease being the number one cause of death in many parts of the world, and the well-established relationship between cardiovascular events and inflammation, the COLCOT (Colchicine Cardiovascular Outcome Trial) was conducted to evaluate the ability of colchicine to lower the risk of subsequent acute coronary events [5].
An old drug dating back to 1500 B.C. to treat joint problems, colchicine has indications for Familial Mediterranean Fever, gout, and pericarditis [6]. The COLCOT trial was conducted in 167 centers, across 12 nations, and was presented at the American Heart Association 2019 Scientific Sessions in Philadelphia, PA.
Study Design:
Secondary Endpoint: components of primary composite of CV death, cardiac arrest, MI or stroke.
Inclusion criteria:
Results
The mean age was 61 years. Overall, 20% of total participants were female, and 20% of total participants were diabetic. A total of 93% of patients underwent PCI during the index MI. ASA, another antiplatelet drug, and statin were taken by 98.8%, 97.9%, and 99% respectively.
The primary efficacy outcome demonstrated:
Discussion
Low dose colchicine has shown reduction in cardiac death, myocardial infarction, stroke, and urgent hospitalization when compared to placebo (5.5% versus 7.1% respectively). The side effects of colchicine were largely GI -related symptoms. The colchicine group did not have significantly more patients with diarrhea when compared to placebo (9.7% versus 8.9%). Despite the positive effect of colchicine on preventing recurrent major cardiovascular events in the COLCOT trial, more studies are needed before it can be recommended as part of the routine post–MI medical therapy. The positive effect of colchicine may be related to the overwhelming number of patients already on appropriate medical therapy (statins and antiplatelet therapy) and having received appropriate revascularization (93% of patients from each group). In addition, the relatively short follow up time of 23 months did not allow for comprehensive review of risks versus benefits of long term use of colchicine. Therefore, further larger studies with longer follow up time are needed to assess the side effects of colchicine and to study colchicine’s effect in other high risk patients without recent ACS.
Clinical Implications
The findings from the COLOCT trial do not come as a surprise for many physicians, as the harmful effects of inflammation are well known and manifest in several ways. Reduction of inflammation has been the target of many recent atherosclerosis therapeutic regimens [7]. Although colchicine has shown promise in its ability to mitigate inflammation and reduce events post MI [8], the safety of its long term use for cardiac indications remains in question [9] and further studies are needed to establish a definitive role for its use in atherosclerosis.
References
Visiting Student
University of South Alabama
Mobile, AL
Landai Nguyen, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Inflammation has long been known to raise the risk of plaque rupture, leading to acute coronary syndromes [1]. Multiple trials have been done in the past to show the benefits of inflammation reduction [2]. The CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) trial showed that canakinumab can lower cardiovascular events when compared to placebo, due to inhibition of interleukin 1B [3]. The LoDoCo (Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease) trial demonstrated that patients receiving 0.5 mg daily of colchicine had fewer cardiovascular events than patients who did not receive colchicine [4]. Thus, with heart disease being the number one cause of death in many parts of the world, and the well-established relationship between cardiovascular events and inflammation, the COLCOT (Colchicine Cardiovascular Outcome Trial) was conducted to evaluate the ability of colchicine to lower the risk of subsequent acute coronary events [5].
An old drug dating back to 1500 B.C. to treat joint problems, colchicine has indications for Familial Mediterranean Fever, gout, and pericarditis [6]. The COLCOT trial was conducted in 167 centers, across 12 nations, and was presented at the American Heart Association 2019 Scientific Sessions in Philadelphia, PA.
Study Design:
- Randomized, double-blind clinical trial
- Median time of follow up: 22.6 months
- 4745 patients were recruited within 30 days of having a myocardial infarction
- 2366 patients received 0.5 mg of colchicine daily
- 2379 patients received placebo daily
Secondary Endpoint: components of primary composite of CV death, cardiac arrest, MI or stroke.
Inclusion criteria:
- Men and women over 18 years old
- Myocardial infarction within 30 days of enrollment
- Completed any planned revascularization procedures
- On guideline-directed medical treatment, including statin
- LV EF < 35%
- Stroke within 3 months
- Type 2 MI
- CABG (within previous 3 years or planned)
- IBS or chronic diarrhea
- Neuromuscular disease
- Nontransient creatinine kinase level > 3x ULN
- Nontransient hematologic abnormalities
- Significant hepatic or renal disease
- Chronic corticosteroid therapy
- Drug/EtOH abuse
- Sensitivity to colchicine
Results
The mean age was 61 years. Overall, 20% of total participants were female, and 20% of total participants were diabetic. A total of 93% of patients underwent PCI during the index MI. ASA, another antiplatelet drug, and statin were taken by 98.8%, 97.9%, and 99% respectively.
The primary efficacy outcome demonstrated:
- Cardiovascular death, myocardial infarction, stroke, resuscitated cardiac arrest, or urgent hospitalization for unstable angina leading to revascularization happened in 5.5% of the colchicines group, versus 7.1% of the placebo group (p = 0.02)
- Cardiovascular death: 0.8% in the colchicine group versus 1% of the placebo group
- Stroke: 0.2% in the colchicine group versus 0.8% in the placebo group
- Urgent hospitalization for revascularization: 1.1% in the colchicine group versus 2.1% in the placebo group
- Infection: 2.2% in the colchicine group versus 1.6% in the placebo group
- Diarrhea: 9.7% in the colchicine group versus 8.9% in the placebo group
Discussion
Low dose colchicine has shown reduction in cardiac death, myocardial infarction, stroke, and urgent hospitalization when compared to placebo (5.5% versus 7.1% respectively). The side effects of colchicine were largely GI -related symptoms. The colchicine group did not have significantly more patients with diarrhea when compared to placebo (9.7% versus 8.9%). Despite the positive effect of colchicine on preventing recurrent major cardiovascular events in the COLCOT trial, more studies are needed before it can be recommended as part of the routine post–MI medical therapy. The positive effect of colchicine may be related to the overwhelming number of patients already on appropriate medical therapy (statins and antiplatelet therapy) and having received appropriate revascularization (93% of patients from each group). In addition, the relatively short follow up time of 23 months did not allow for comprehensive review of risks versus benefits of long term use of colchicine. Therefore, further larger studies with longer follow up time are needed to assess the side effects of colchicine and to study colchicine’s effect in other high risk patients without recent ACS.
Clinical Implications
The findings from the COLOCT trial do not come as a surprise for many physicians, as the harmful effects of inflammation are well known and manifest in several ways. Reduction of inflammation has been the target of many recent atherosclerosis therapeutic regimens [7]. Although colchicine has shown promise in its ability to mitigate inflammation and reduce events post MI [8], the safety of its long term use for cardiac indications remains in question [9] and further studies are needed to establish a definitive role for its use in atherosclerosis.
References
- Boyle JJ. Association of Coronary Plaque Rupture and Atherosclerotic Inflammation. J Pathol, 181 (1), 93-9.
- Deepak L Bhatt. Anti-inflammatory Agents and Antioxidants as a Possible "Third Great Wave" in Cardiovascular Secondary Prevention. Am J Cardiol, 101 (10A), 4D-13D.
- Ridker PM, Everett BM, Thuren Tom, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377: 1119 – 1131.
- Nidorf SM, et al. Low Dose Colchicine for Secondary Prevention of Cardiovascular disease. J. Am. Coll, Cardiol. 2017. 61: 404 – 410.
- Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine After Myocardial Infarction. N Engl J Med 2019;381:2497-505.
- Nerlekar N, Beale A, Harper RW. Colchicine--a Short History of an Ancient Drug. Med J Aust, 201 (11), 687-8.
- Charo IF, Taub R. Anti-inflammatory Therapeutics for the Treatment of Atherosclerosis. Nat Rev Drug Discov, 10 (5), 365-76.
- Nidorf SM, Thompson PL. Why Colchicine Should Be Considered for Secondary Prevention of Atherosclerosis: An Overview. Clin Ther, 41 (1), 41-48.
- Hemkens LG, Ewald H, Gloy VL, et al. Cardiovascular Effects and Safety of Long-Term Colchicine Treatment: Cochrane Review and Meta-Analysis. Heart, 102 (8), 590-6.
Visiting Student
University of South Alabama
Mobile, AL
Landai Nguyen, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Quadricuspid Aortic Valve..Associated ASD!
Description
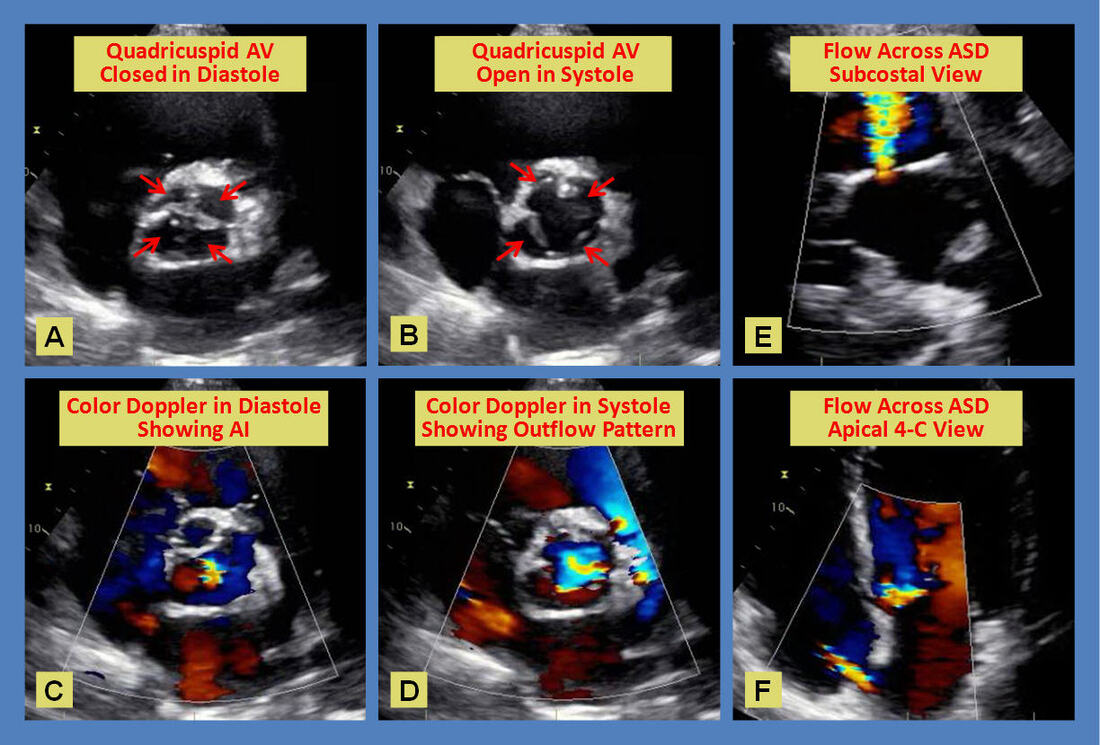
The figure above shows a 2-dimensional parasternal short axis view of a quadricuspid aortic valve in closed (A) and open (B) position with arrows pointing to the individual cusps. Note the sclerosis affecting the coapt points. Color flow Doppler across the valve in diastole (C) shows a central jet of mild aortic regurgitation, and the outflow pattern across the valve in systole (D). The subcostal view (E) and the apical 4-chamber view (F) both demonstrate spontaneous left to right shunting across the interatrial septum consistent with an ostium secundum atrial septal defect.
While bicuspid aortic valve is the most common anomaly of the aortic valve, with a global incidence of 1.3%, followed by unicuspid aortic valve, quadricuspid aortic valve is a rare finding, with a reported incidence of less than 0.05%, which is least among the abnormal variants of aortic valve [1]. The first case of quadricuspid aortic valve was reported in 1862 by Belington [2].
Hurwitz and Roberts [3] classified quadricuspid aortic valves into seven types based on variations in the size of the cusps. Type B, which is composed of three cusps of same size and one smaller cusp, as shown in the figure above, is the most prevalent type (41%) followed by type A (32%) in which all four cusps are of same size. Prevalence of aortic regurgitation is 77% in type A and 60% in type B.
Although quadricuspid aortic valve is often an isolated anomaly, coexistent cardiac malformations have been reported, especially coronary anomalies [4]. Knowledge of the coronary anatomy is very important to avoid damage to anomalous coronary ostia during valve surgery [5, 6]. Sudden cardiac death has been reported in young patient with quadricuspid aortic valve with complete isolation of the left coronary artery by an adherent aortic cusp leading to myocardial infarction [7].
Other reported cardiac abnormalities associated with quadricuspid aortic valve include VSD [8], PDA [9], Pulmonary stenosis [10], complete Heart Block [11], dilated and noncompaction cardiomyopathies [12, 13], and hypertrophic cardiomyopathy [14]. Several case reports have also been published establishing an association of quadricuspid aortic valve with a patent foramen ovale and an atrial septal defect, as in the figure above [15 – 19]. Cases of infective endocarditis [20], ischemic stroke [21], aortic dissection [22] and left ventricular hemangioma [23] have also been reported in patients with quadricuspid aortic valve.
The mean age of presentation in patients with a quadricuspid valve is 50.7 years, with a slight male predominance with a male to female ratio of 1.6 [24]. Most common associated functional abnormality is aortic regurgitation (75% of the cases), followed by combined aortic regurgitation and aortic stenosis (8% of the cases), while isolated aortic stenosis is rare (0.7% of the cases). Approximately 16% of the cases of quadricuspid valve are normally functioning with no associated functional abnormalities.
Although echocardiography is the gold standard for diagnosing quadricuspid aortic valve, occasional cases may be missed [25]. Advanced imaging has been shown to help better visualize quadricuspid valves including 3-dimensional (3-D) transthoracic echocardiography [26], transesophageal echocardiography (TEE) [27], intraoperative TEE [28] and 3-D TEE [29]. Visualization using cardiac magnetic resonance imaging [30] and cardiac computed tomography [31] can be diagnostic or supplementary to echocardiography. Multimodality imaging is often required to better visualize a quadricuspid valve and characterize associated anomalies [32].
Quadricuspid aortic valve is a rare congenital heart defect with a high potential for serious complications. Patients with this condition should be carefully evaluated for associated anomalies and require close follow up [1]. Treatment of the quadricuspid aortic valve is dependent on associated anomalies and severity of the valve dysfunction (often aortic regurgitation). When there is need for surgical intervention, treatment may entail aortic valve replacement [33], aortic valve repair [34], and more recently, transcatheter aortic valve replacement (TAVR) [35]. Close post-operative long term follow-up is required given the ongoing risk of aortic root and ascending aorta dilatation [36].
References:
Authors:
Sagar Kumar, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Mahmoud N. Hamad
Medical Student
Royal College of Surgeons
Dublin, Ireland
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The figure above shows a 2-dimensional parasternal short axis view of a quadricuspid aortic valve in closed (A) and open (B) position with arrows pointing to the individual cusps. Note the sclerosis affecting the coapt points. Color flow Doppler across the valve in diastole (C) shows a central jet of mild aortic regurgitation, and the outflow pattern across the valve in systole (D). The subcostal view (E) and the apical 4-chamber view (F) both demonstrate spontaneous left to right shunting across the interatrial septum consistent with an ostium secundum atrial septal defect.
While bicuspid aortic valve is the most common anomaly of the aortic valve, with a global incidence of 1.3%, followed by unicuspid aortic valve, quadricuspid aortic valve is a rare finding, with a reported incidence of less than 0.05%, which is least among the abnormal variants of aortic valve [1]. The first case of quadricuspid aortic valve was reported in 1862 by Belington [2].
Hurwitz and Roberts [3] classified quadricuspid aortic valves into seven types based on variations in the size of the cusps. Type B, which is composed of three cusps of same size and one smaller cusp, as shown in the figure above, is the most prevalent type (41%) followed by type A (32%) in which all four cusps are of same size. Prevalence of aortic regurgitation is 77% in type A and 60% in type B.
Although quadricuspid aortic valve is often an isolated anomaly, coexistent cardiac malformations have been reported, especially coronary anomalies [4]. Knowledge of the coronary anatomy is very important to avoid damage to anomalous coronary ostia during valve surgery [5, 6]. Sudden cardiac death has been reported in young patient with quadricuspid aortic valve with complete isolation of the left coronary artery by an adherent aortic cusp leading to myocardial infarction [7].
Other reported cardiac abnormalities associated with quadricuspid aortic valve include VSD [8], PDA [9], Pulmonary stenosis [10], complete Heart Block [11], dilated and noncompaction cardiomyopathies [12, 13], and hypertrophic cardiomyopathy [14]. Several case reports have also been published establishing an association of quadricuspid aortic valve with a patent foramen ovale and an atrial septal defect, as in the figure above [15 – 19]. Cases of infective endocarditis [20], ischemic stroke [21], aortic dissection [22] and left ventricular hemangioma [23] have also been reported in patients with quadricuspid aortic valve.
The mean age of presentation in patients with a quadricuspid valve is 50.7 years, with a slight male predominance with a male to female ratio of 1.6 [24]. Most common associated functional abnormality is aortic regurgitation (75% of the cases), followed by combined aortic regurgitation and aortic stenosis (8% of the cases), while isolated aortic stenosis is rare (0.7% of the cases). Approximately 16% of the cases of quadricuspid valve are normally functioning with no associated functional abnormalities.
Although echocardiography is the gold standard for diagnosing quadricuspid aortic valve, occasional cases may be missed [25]. Advanced imaging has been shown to help better visualize quadricuspid valves including 3-dimensional (3-D) transthoracic echocardiography [26], transesophageal echocardiography (TEE) [27], intraoperative TEE [28] and 3-D TEE [29]. Visualization using cardiac magnetic resonance imaging [30] and cardiac computed tomography [31] can be diagnostic or supplementary to echocardiography. Multimodality imaging is often required to better visualize a quadricuspid valve and characterize associated anomalies [32].
Quadricuspid aortic valve is a rare congenital heart defect with a high potential for serious complications. Patients with this condition should be carefully evaluated for associated anomalies and require close follow up [1]. Treatment of the quadricuspid aortic valve is dependent on associated anomalies and severity of the valve dysfunction (often aortic regurgitation). When there is need for surgical intervention, treatment may entail aortic valve replacement [33], aortic valve repair [34], and more recently, transcatheter aortic valve replacement (TAVR) [35]. Close post-operative long term follow-up is required given the ongoing risk of aortic root and ascending aorta dilatation [36].
References:
- Tsang MY, Abudiab MM, Ammash NM, et al. Quadricuspid Aortic Valve: Characteristics, Associated Structural Cardiovascular Abnormalities, and Clinical Outcomes. Circulation. 2016 Jan 19;133(3):312-9.
- Balington J, quoted by Robicsek F, Sanger PW, Daugherty HK, Montgomery CC. Congenital quadricuspid aortic valve with displacement of the left coronary orifice. Am J Cardiol 1969; 23:288–90.
- Hurwitz LE, Roberts WC. Quadricuspid semilunar valve. Am J Cardiol. 1973 May;31(5):623-6.
- Gulyasy B, Lopez-Candales A, Reis SE, et al. Quadricuspid aortic valve: an unusual echocardiography finding and a review of the literature; Int. J. Cardiol., 132 (2009), pp. 68-71
- Braga A, Marques M, Abecasis M, et al. Quadricuspid aortic valve associated with two left main coronary ostia. J Card Surg. 2018 Nov;33(11):746-747.
- Kim DY, Kim HW. Single coronary ostium in a patient with quadricuspid aortic valve combined with aneurysmal ascending aortic dilatation. J Cardiothorac Surg. 2017 Jul 24;12(1):59
- Kurosawa H, Wagenaar SS, Becker AE. Sudden death in a youth. A case of quadricuspid aortic valve with isolation of origin of left coronary artery. Br Heart J. 1981 Aug;46(2):211-5.
- Demirkol S, Balta S, Arslan Z, Unlu M, et al. Association of quadricuspid aortic valve and ventricular septal defect in a patient who had undergone atrial septal defect surgery. Kardiol Pol. 2013;71(5):546.
- Seol SH, Kim U, Cho HJ, et al. Quadricuspid aortic valve with patent ductus arteriosus. Tex Heart Inst J. 2010;37(6):726-7.
- Possati F, Calafiore AM, Di Giammarco G, et al. [Quadricuspid aortic valve and pulmonary valve stenosis. A rare combination in the adult]. [Article in Italian]. Minerva Cardioangiol. 1984 Nov;32(11):815-8.
- Moreno R, Zamorano J, De Marco E, et al. Congenital quadricuspid aortic valve associated with congenital complete heart block. Eur J Echocardiogr. 2002 Sep;3(3):236-7.
- Tsujimoto S, Motohiro M, Kamihata H, et al. Quadricuspid aortic valve associated with idiopathic dilated cardiomyopathy: A case report. J Cardiol Cases. 2014 Apr 18;9(6):233-235.
- Doğan M, Bağbancı H, Türkvatan A, et al. Quadricuspid aortic valve associated with persistent left superior vena cava and right ventricular noncompaction cardiomyopathy. Turk Kardiyol Dern Ars. 2013 Jul;41(5):459.
- Janssens U, Klues HG, Hanrath P. Congenital quadricuspid aortic valve anomaly associated with hypertrophic non-obstructive cardiomyopathy: a case report and review of the literature. Heart. 1997 Jul;78(1):83-7.
- Kosior DA, Piatkowski R, Bakoń L. Quadricuspid aortic valve with mild aortic regurgitation and persistent foramen ovale: a multimodality imaging of rare concomitant findings. J Heart Valve Dis. 2013 Nov;22(6):878-9.
- Vohra RK, Singh H, Siu BL, et al. A quadricuspid aortic valve with atrial septal defect. Echocardiography. 2006 Nov;23(10):865-8.
- Saito N, Yoshimura T, Miyatsu K, et al. [Quadricuspid Aortic Valve with Atrial Septal Defect;Report of a Case]. [Article in Japanese]. Kyobu Geka. 2017 Mar;70(3):203-206.
- Garg A, Garg S, Agrawal D, et al. Quadricuspid Aortic Valve With Ostium Secundum Atrial Septal Defect. CASE (Phila). 2019 May 17;3(4):138-140.
- Sousa L, Pinto F, Nogueira G, et al. Quadricuspid aortic valve and atrial septal defect. [Article in English, Portuguese]. Rev Port Cardiol. 2001 Mar;20(3):329-30.
- Jackson C, Sarwar T, Hwang I, et al. Quadricuspid aortic valve infective endocarditis. J Clin Ultrasound. 2018 Feb;46(2):145-148.
- Krisper M, Köhncke C, Escher F, Morris et al. A Patient with Quadricuspid Aortic Valve and Ischemic Stroke. J Heart Valve Dis. 2016 Jul;25(4):456-458.
- Klassen SL, Hutchison SJ. Quadricuspid Aortic Valvulopathy and Acute Type A Aortic Dissection. Aorta (Stamford). 2019 Jun;7(3):93-95.
- Sun Z, Wang B, Li H, et al. Left ventricular hemangioma and quadricuspid aortic valve: a rare combination. Int J Cardiovasc Imaging. 2020 Jan 3.
- Tutarel O. The quadricuspid aortic valve: a comprehensive review. J Heart Valve Dis. 2004 Jul;13(4):534-7.
- Dencker M, Stagmo M. Quadricuspid aortic valve not discovered by transthoracic echocardiography. Cardiovasc Ultrasound. 2006 Nov 7;4:41.
- Acar E, Sahin T, Yılmaz I, et al. Quadricuspid Aortic Valve Visualized by Three-Dimensional Transthoracic Echocardiography. Case Rep Cardiol. 2011; 2011: 345721.
- Nikdoust F, Sadeghian H, Eslami B, et al. Quadricuspid Aortic Valve Diagnosed by Transesophageal Echocardiography: A Case Report. J Tehran Heart Cent. 2010 Spring; 5(2): 95–97.
- Xiao Z, Meng W, Zhang E. Quadricuspid aortic valve by using intraoperative transesophageal echocardiography. Cardiovasc Ultrasound. 2010; 8: 36.
- Kanda H, Kunisawa T, Iida T, Kanao et al. Quadricuspid aortic valve detected by three-dimensional transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2015;29(3):e33-5.
- Khan SK, Tamin SS, Araoz PA. Quadricuspid aortic valve by cardiac magnetic resonance imaging: a case report and review of the literature. J Comput Assist Tomogr. 2011 Sep-Oct;35(5):637-41.
- Karlsberg DW, Elad Y, Kass RM, et al. Quadricuspid aortic valve defined by echocardiography and cardiac computed tomography. Clin Med Insights Cardiol. 2012;6:41-4.
- Moorthy N, Kapoor A, Kumar S, et al. Quadricuspid aortic valve: multimodality imaging. Pediatr Cardiol. 2013 Apr;34(4):1059-61.
- Arumugam S, Lam KY, Akca F, et al. Quadricuspid aortic valve replacement. J Card Surg. 2017 Sep;32(9):579-580.
- Mastrobuoni S, Aphram G, Tamer S, et al. Quadricuspid aortic valve repair. Ann Cardiothorac Surg. 2019 May;8(3):433-435.
- Ibrahim M, Wattanakit K, Barzallo M, et al. Quadricuspid Aortic Valve Stenosis: Expanding Our Experience in Transcatheter Aortic Valve Implantation. J Invasive Cardiol. 2018 Mar;30(3):E27.
- Idrees JJ, Roselli EE, Arafat A, et al. Outcomes after repair or replacement of dysfunctional quadricuspid aortic valve. J Thorac Cardiovasc Surg. 2015 Jul;150(1):79-82.
Authors:
Sagar Kumar, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Mahmoud N. Hamad
Medical Student
Royal College of Surgeons
Dublin, Ireland
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL