July 2019 Issue
ISSN 2689-291X
ISSN 2689-291X
Challenging Images
Ventricular Diverticulum! Beyond The Colon!
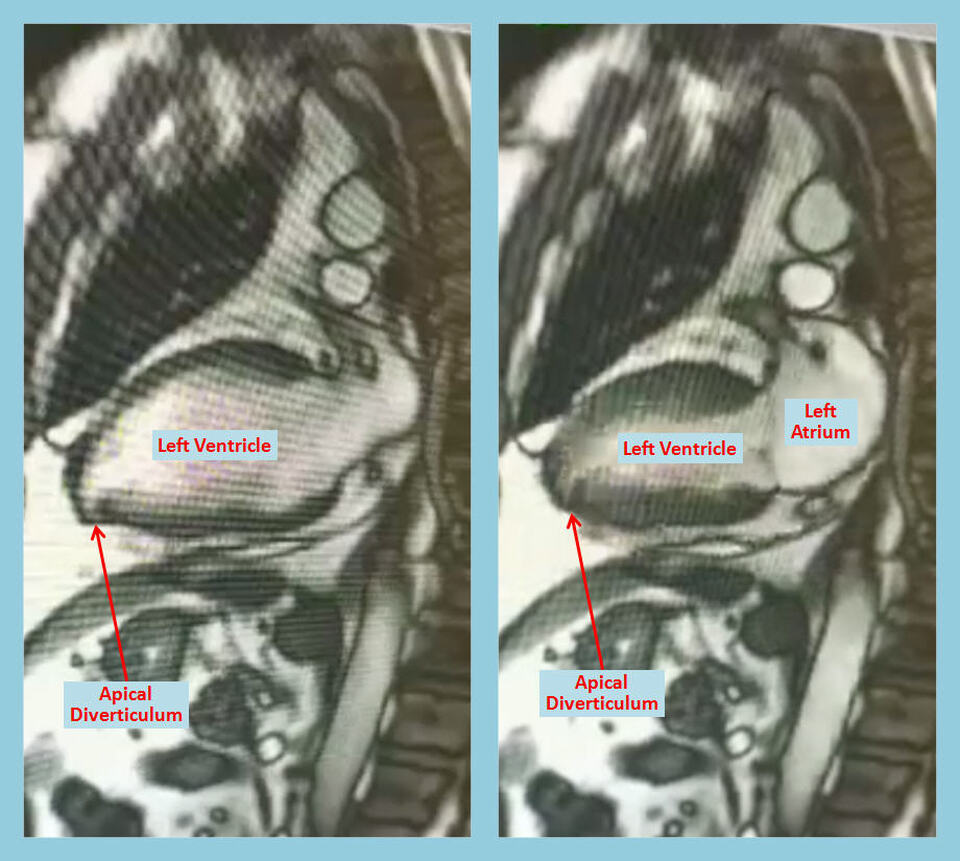
Description
Diverticulum, aneurysm and pseudoaneurysm are some left ventricular outpouchings that are increasingly detected on imaging with the advent of recent imaging techniques [1]. Management depends on proper distinction among these entities as outcomes differ drastically; unfortunately, such distinction may be challenging [2]. Echocardiography remains the initial test of choice in the diagnosis and characterization of these lesions, but it is operator dependent and sometimes can be technically limited [3]. Cardiac Computed Tomography (CCT), Cardiac Magnetic Resonance imaging (CMR) and left ventriculography may help enhance the diagnosis and characterization of these outpouchings [4].
Left ventricular diverticulum is an outpouching that contains all three layers such as endocardium, myocardium, and pericardium and displays synchronous contractility. As per the earlier studies reported a prevalence of 0.4%, however it has increased prevalence of 2.2% after the usage of multi-detector computed tomography angiography [5]. They may be congenital or acquired, former being more common. Most common congenital causes of left diverticulum is excessive primordial cell stimulation, in utero viral infection, muscle and connective tissue defects, and midline defects in association with other malformations, as part of Cantrell Syndrome [6].
These diverticula are often asymptomatic and may be found incidentally during diagnostic work up for other reasons. Their size may vary from as small as 0.5 cm in diameter to as large as 8-9 cm [7]. Embolism, rupture, thrombosis, and ventricular arrhythmias can complicate these diverticula, however true incidence of these complications remains unknown [8]. Spontaneous regression is seen in few cases and their size may not change over a considerable period of time suggesting a benign course. Close clinical follow up is usually sufficient and further management should be based on associated abnormalities and potential complications.
CMR allows anatomical and functional evaluation of left ventriculum diverticulum, along with tissue characterization, which carries therapeutic and prognostic implications [9]. Diverticulum contains all myocardial layers, and CMR is very helpful in tissue characterization, distinguishing among pericardium, thrombus and the myocardium. Although such characterization may be difficult on conventional CMR in thinned out and scarred or infracted myocardium, such imaging remains crucial in planning surgical intervention as it can delineate the relationship of the diverticulum to different structures like the mitral valve [10].
References:
Authors:
Nikky Bardia, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Pavani Kolakalapudi, M.D.
Staff Cardiologist
The Heart Center of Northeast Georgia Medical Center
Braselton, GA
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Diverticulum, aneurysm and pseudoaneurysm are some left ventricular outpouchings that are increasingly detected on imaging with the advent of recent imaging techniques [1]. Management depends on proper distinction among these entities as outcomes differ drastically; unfortunately, such distinction may be challenging [2]. Echocardiography remains the initial test of choice in the diagnosis and characterization of these lesions, but it is operator dependent and sometimes can be technically limited [3]. Cardiac Computed Tomography (CCT), Cardiac Magnetic Resonance imaging (CMR) and left ventriculography may help enhance the diagnosis and characterization of these outpouchings [4].
Left ventricular diverticulum is an outpouching that contains all three layers such as endocardium, myocardium, and pericardium and displays synchronous contractility. As per the earlier studies reported a prevalence of 0.4%, however it has increased prevalence of 2.2% after the usage of multi-detector computed tomography angiography [5]. They may be congenital or acquired, former being more common. Most common congenital causes of left diverticulum is excessive primordial cell stimulation, in utero viral infection, muscle and connective tissue defects, and midline defects in association with other malformations, as part of Cantrell Syndrome [6].
These diverticula are often asymptomatic and may be found incidentally during diagnostic work up for other reasons. Their size may vary from as small as 0.5 cm in diameter to as large as 8-9 cm [7]. Embolism, rupture, thrombosis, and ventricular arrhythmias can complicate these diverticula, however true incidence of these complications remains unknown [8]. Spontaneous regression is seen in few cases and their size may not change over a considerable period of time suggesting a benign course. Close clinical follow up is usually sufficient and further management should be based on associated abnormalities and potential complications.
CMR allows anatomical and functional evaluation of left ventriculum diverticulum, along with tissue characterization, which carries therapeutic and prognostic implications [9]. Diverticulum contains all myocardial layers, and CMR is very helpful in tissue characterization, distinguishing among pericardium, thrombus and the myocardium. Although such characterization may be difficult on conventional CMR in thinned out and scarred or infracted myocardium, such imaging remains crucial in planning surgical intervention as it can delineate the relationship of the diverticulum to different structures like the mitral valve [10].
References:
- Malakan Rad E, Awad S, Hijazi ZM. Congenital left ventricular outpouchings: a systematic review of 839 cases and introduction of a novel classification after two centuries. Congenit Heart Dis. 2014 Nov-Dec;9(6):498-511.
- Halpern L, Garabedian C, Worrall NK. Congenital Ventricular Diverticulum or Aneurysm: A Difficult Diagnosis to Make. Case Rep Cardiol. 2018 Nov 11;2018:5839432.
- Gadde S, Omar B. Chest Pain With Apical Diverticulum in the Absence of Coronary Disease: Case Report and Review of the Literature. Cardiol Res. 2015 Dec;6(6):352-356.
- Sharma A, Kumar S. Overview of left ventricular outpouchings on cardiac magnetic resonance imaging. Cardiovasc Diagn Ther. 2015 Dec;5(6):464-70.
- Dwivedi AN, Thangiah AG, Rai M, et al. Computed tomographic features of congenital left ventricular diverticulum. J Clin Imaging Sci. 2012;2:48.
- Ojha V, Sh C, Ganga KP, et al. Congenital left ventricular diverticulum in Pentalogy of Cantrell: Puzzle solved on dual source CT. Ann Thorac Surg. 2019 Mar 25. pii: S0003-4975(19)30406-0.
- Makaryus AN1, Peters MR. Congenital left ventricular diverticulum diagnosed by 64-detector CT imaging. J Invasive Cardiol. 2008 Jul;20(7):372-3.
- Nakanishi K, Sakurai S, Kawabata T, et al. Cerebral thromboembolism in a patient awaiting surgery for left ventricular diverticulum. Interact Cardiovasc Thorac Surg. 2011 Feb;12(2):319-20
- Aquaro GD, Di Bella G, Strata E, et al. Cardiac magnetic resonance findings in isolated congenital left ventricular diverticuli. Int J Cardiovasc Imaging. 2007 Feb;23(1):43-7.
- Pitsis AA, Visouli AN, Kelpis TG, et al. Diverticulum of the left ventricle: etiology and surgical treatment. Heart Surg Forum. 2008;11(2):E75-7.
Authors:
Nikky Bardia, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Pavani Kolakalapudi, M.D.
Staff Cardiologist
The Heart Center of Northeast Georgia Medical Center
Braselton, GA
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Let Atrial Appendage Thrombus! On TTE!
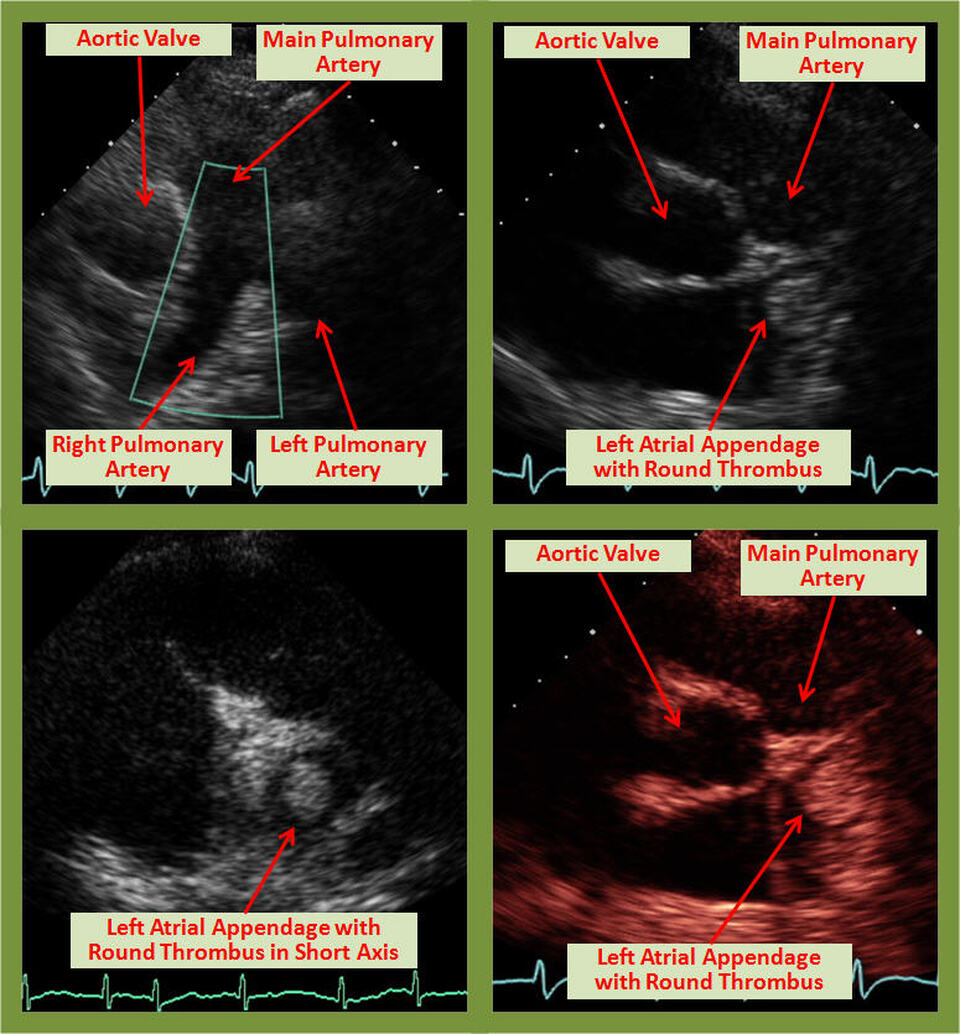
Description
The left atrial appendage (LAA) is rarely visualized on transthoracic echocardiography (TTE) with sufficient clarity to exclude thrombi. Usually transesophageal echocardiography (TEE) is required for this purpose. The image shows a surprisingly clear LAA thrombus in the body of the LAA in the long axis (including B-mode color) and short axis TTE views.
Introduction
The left atrial appendage (LAA) is a long, tubular, hooked structure with a narrow base which lies within the pericardium close to the free wall of left ventricle. It is the remnant of the embryonic left atrium which develops during the third week of gestation and has a trabecular surface [1]. The smooth walled left atrial cavity develops from the extension of the pulmonary veins.
Function
The LAA serves as a decompression chamber during left ventricular (LV) systole or in certain circumstances when the left atrial pressure is elevated [2]. Due to the proximity of the LAA to the free wall of the left ventricle, the emptying and filling of the LAA is affected by the LV. As the LV dilates during diastole, it fills the intrapericardial space which helps in appendageal emptying by compressing the inferomedial wall of the LAA. This finding also supports the higher incidence of stroke-related events in patients with atrial fibrillation who have LV dysfunction [3].
Evaluation
The LAA has a predilection for thrombus formation due to its shape and structure, particularly in patients with atrial fibrillation who are not on anticoagulation therapy. LAA is best evaluated by transesophageal echocardiography (TEE), [4] which permits a detailed examination of its structure, whereas TTE merely shows an outline of the LAA. However, occasionally thrombus in the LAA can be visualized on transthoracic echocardiography (TTE) as in the images above, especially large thrombi that extend into the body of the left atrium [5]. Similarly, in one multicenter study, there were two atrial appendage thrombi identified on TTE using harmonic imaging and left-sided echocardiographic contrast [6]. Based on a study in patients undergoing mitral valve surgery, TEE was noted to be 93% sensitive and 100% specific for thrombus detection, while the sensitivity of TTE was only 53% [7].
CONCLUSION
LAA is a unique structure which acts as a decompression chamber for the left atrium and ventricle. Due to its anatomy and physiology, it has the tendency for thrombus formation. TEE is the preferred diagnostic tool for the evaluation of LAA and detection of thrombi within it. However, TTE may have a role in detection of LAA thrombi using different techniques such as harmonic imaging and left-sided echocardiographic contrast.
References:
Authors:
Kulwant Bath, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The left atrial appendage (LAA) is rarely visualized on transthoracic echocardiography (TTE) with sufficient clarity to exclude thrombi. Usually transesophageal echocardiography (TEE) is required for this purpose. The image shows a surprisingly clear LAA thrombus in the body of the LAA in the long axis (including B-mode color) and short axis TTE views.
Introduction
The left atrial appendage (LAA) is a long, tubular, hooked structure with a narrow base which lies within the pericardium close to the free wall of left ventricle. It is the remnant of the embryonic left atrium which develops during the third week of gestation and has a trabecular surface [1]. The smooth walled left atrial cavity develops from the extension of the pulmonary veins.
Function
The LAA serves as a decompression chamber during left ventricular (LV) systole or in certain circumstances when the left atrial pressure is elevated [2]. Due to the proximity of the LAA to the free wall of the left ventricle, the emptying and filling of the LAA is affected by the LV. As the LV dilates during diastole, it fills the intrapericardial space which helps in appendageal emptying by compressing the inferomedial wall of the LAA. This finding also supports the higher incidence of stroke-related events in patients with atrial fibrillation who have LV dysfunction [3].
Evaluation
The LAA has a predilection for thrombus formation due to its shape and structure, particularly in patients with atrial fibrillation who are not on anticoagulation therapy. LAA is best evaluated by transesophageal echocardiography (TEE), [4] which permits a detailed examination of its structure, whereas TTE merely shows an outline of the LAA. However, occasionally thrombus in the LAA can be visualized on transthoracic echocardiography (TTE) as in the images above, especially large thrombi that extend into the body of the left atrium [5]. Similarly, in one multicenter study, there were two atrial appendage thrombi identified on TTE using harmonic imaging and left-sided echocardiographic contrast [6]. Based on a study in patients undergoing mitral valve surgery, TEE was noted to be 93% sensitive and 100% specific for thrombus detection, while the sensitivity of TTE was only 53% [7].
CONCLUSION
LAA is a unique structure which acts as a decompression chamber for the left atrium and ventricle. Due to its anatomy and physiology, it has the tendency for thrombus formation. TEE is the preferred diagnostic tool for the evaluation of LAA and detection of thrombi within it. However, TTE may have a role in detection of LAA thrombi using different techniques such as harmonic imaging and left-sided echocardiographic contrast.
References:
- Sadler TW. Cardiovascular system. In: Langman J, ed. Langman’s medical embryology, 6th ed. Baltimore: Williams and Wilkins, 1990:179–227.
- Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart 1999; 82:547.
- The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann Intern Med 1992;116:6–12.
- Agmon Y, Khandheria BK, Gentile F, Seward JB. Echocardiographic assessment of the left atrial appendage. J Am Coll Cardiol 1999; 34:1867.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66.
- Sallach JA, Puwanant S, Drinko JK, et al. Comprehensive left atrial appendage optimization of thrombus using surface echocardiography: the CLOTS multicenter pilot trial. J Am Soc Echocardigr 2009; 22:1165.
- Hwang JJ, Chen JJ, Lin SC, et al. Diagnostic accuracy of transesophageal echocardiography for detecting left atrial thrombi in patients with rheumatic heart disease having undergone mitral valve operations. Am J Cardiol 1993; 72:677.
Authors:
Kulwant Bath, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL