June 2019 Issue
ISSN 2689-291X
ISSN 2689-291X
Challenging Images
Unicuspid Aortic Valve! Not So Common!
Description
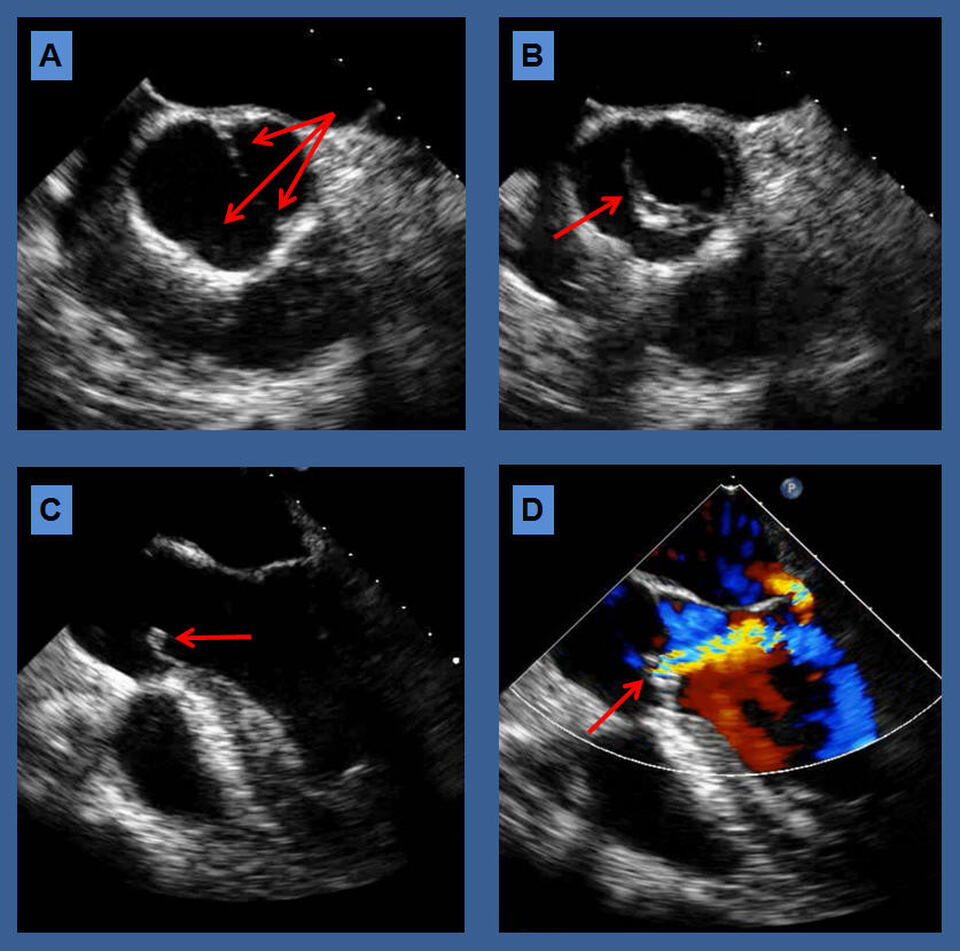
The transesophageal echocardiography images show a unicuspid aortic valve in the short axis view in closed position (A) with arrows pointing at the fused commissures; and in the open position (B) showing the circular eccentric position of the orifice (arrow). The Long axis views [C] show the valve in open position with doming of the cusps (arrow), and color flow Doppler (D) revealing at least moderate aortic insufficiency (arrow) with a trivial amount of late diastolic mitral regurgitation.
INTRODUCTION
Unicuspid aortic valve [UAV] is an extremely rare congenital anomaly which results from failure of the three aortic cusps to separate before birth [1]. It was first reported by Edwards in 1958 [7]. Its estimated annual incidence is about 0.02% in the adult population [8]. However, its incidence is noted to be higher in patients who undergo surgery for isolated aortic stenosis, up to 4-5%.1 It is found predominantly in males with male to female ratio of 4:1.
STRUCTURAL FINDINGS
Aortic valve normally consists of 3 cusps with 3 commissures that develop from embryonic tubercules of the aortic trunk. This aberration of unicuspid valve results from failure of the cusps to separate. Unicuspid aortic valves can be further categorized into two types: Acommissural and Unicommissural. In acommissural valves, there are no commissures or lateral attachments to the aorta; the orifice is very small and appears as a pinhole on imaging [6]. These patient have symptomatic severe aortic stenosis at birth or infancy [3]. On the contrary, in unicommissural valves, there is one lateral commissural attachment to the aorta at the level of the orifice which appears as a slit shaped structure. These patients typically present in the 4th to 6th decade of life [1, 2], but can rarely present at infancy [4].
CLINICAL FEATURES
UAV has a bimodal presentation depending upon the type. Accommissural UAV leads to severe aortic stenosis which presents at birth or soon after. Unicommissural UAV has less severe course and presents in late adulthood. Patients may present with dyspnea, angina, or syncope [8]. Isolated aortic stenosis is the most common valvular abnormality associated with UAV. However, it can also be associated with other abnormalities which include aortic aneurysm [5], aortic regurgitation, aortic dissection, coarctation of the aorta, patent ductus arteriosus [2], and aortic dilation. Patients with Aortic stenosis usually become symptomatic once the transvalvular mean gradient exceeds 40 mmHg, the aortic jet velocity is greater than 4 m/s, and the valve area is less than 1 cm2, irrespective of the commissural type.
MANAGEMENT
UAV can be diagnosed with 2D or 3D TTE or TEE [6], cardiac computed tomography [14], or cardiac magnetic resonance imaging [15]. Transesophageal echocardiography (TEE) is the gold standard for diagnosis with a sensitivity and specificity of 75 and 86% respectively [9]. The UAV has an eccentric “teardrop” opening during systole in a unicommissural UAV [8] due to absence of cusp separation. Current guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA) recommend aortic valve replacement (AVR) for symptomatic severe aortic stenosis (Class IB recommendation). However, AVR in patient with UAV is not recommended especially in the young population, due to higher mortality rates when compared to adults. Re-operations due to patient-prosthesis mismatch and structural valve degeneration is also higher [10]. Alternatively, balloon valvuloplasty, surgical valvotomy, or commissurotomy are the initial treatments of choice. If AVR is needed, the Ross procedure is recommended, in which aortic valve is replaced with patient’s own pulmonic valve, reducing the risk of patient-prosthesis mismatch [11, 12]. The autograft also has some capability to grow along with the patient’s heart. The Ross procedure is technically more challenging, with relatively high mortality, but is considered to be safe in experienced hands [10].
CONCLUSION
In Conclusion, unicuspid aortic valve is a rare congenital malformation that often leads to severe aortic stenosis. It is an important clinical entity that should be in the differential diagnosis of younger patients who present with symptoms of heart failure and with a systolic murmur that suggests aortic stenosis. Many cases are diagnosed peri-operatively. However, with evolving imaging technology, a higher incidence of preoperative diagnosis is expected. TEE has a relatively high specificity and sensitivity for diagnosis. Aortic valve repair with balloon valvuloplasty, surgical valvotomy, or commissurotomy are the initial treatments of choice in the young population and AVR is discouraged until patient is fully grown.
REFERENCES:
Authors:
Kulwant Bath, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The transesophageal echocardiography images show a unicuspid aortic valve in the short axis view in closed position (A) with arrows pointing at the fused commissures; and in the open position (B) showing the circular eccentric position of the orifice (arrow). The Long axis views [C] show the valve in open position with doming of the cusps (arrow), and color flow Doppler (D) revealing at least moderate aortic insufficiency (arrow) with a trivial amount of late diastolic mitral regurgitation.
INTRODUCTION
Unicuspid aortic valve [UAV] is an extremely rare congenital anomaly which results from failure of the three aortic cusps to separate before birth [1]. It was first reported by Edwards in 1958 [7]. Its estimated annual incidence is about 0.02% in the adult population [8]. However, its incidence is noted to be higher in patients who undergo surgery for isolated aortic stenosis, up to 4-5%.1 It is found predominantly in males with male to female ratio of 4:1.
STRUCTURAL FINDINGS
Aortic valve normally consists of 3 cusps with 3 commissures that develop from embryonic tubercules of the aortic trunk. This aberration of unicuspid valve results from failure of the cusps to separate. Unicuspid aortic valves can be further categorized into two types: Acommissural and Unicommissural. In acommissural valves, there are no commissures or lateral attachments to the aorta; the orifice is very small and appears as a pinhole on imaging [6]. These patient have symptomatic severe aortic stenosis at birth or infancy [3]. On the contrary, in unicommissural valves, there is one lateral commissural attachment to the aorta at the level of the orifice which appears as a slit shaped structure. These patients typically present in the 4th to 6th decade of life [1, 2], but can rarely present at infancy [4].
CLINICAL FEATURES
UAV has a bimodal presentation depending upon the type. Accommissural UAV leads to severe aortic stenosis which presents at birth or soon after. Unicommissural UAV has less severe course and presents in late adulthood. Patients may present with dyspnea, angina, or syncope [8]. Isolated aortic stenosis is the most common valvular abnormality associated with UAV. However, it can also be associated with other abnormalities which include aortic aneurysm [5], aortic regurgitation, aortic dissection, coarctation of the aorta, patent ductus arteriosus [2], and aortic dilation. Patients with Aortic stenosis usually become symptomatic once the transvalvular mean gradient exceeds 40 mmHg, the aortic jet velocity is greater than 4 m/s, and the valve area is less than 1 cm2, irrespective of the commissural type.
MANAGEMENT
UAV can be diagnosed with 2D or 3D TTE or TEE [6], cardiac computed tomography [14], or cardiac magnetic resonance imaging [15]. Transesophageal echocardiography (TEE) is the gold standard for diagnosis with a sensitivity and specificity of 75 and 86% respectively [9]. The UAV has an eccentric “teardrop” opening during systole in a unicommissural UAV [8] due to absence of cusp separation. Current guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA) recommend aortic valve replacement (AVR) for symptomatic severe aortic stenosis (Class IB recommendation). However, AVR in patient with UAV is not recommended especially in the young population, due to higher mortality rates when compared to adults. Re-operations due to patient-prosthesis mismatch and structural valve degeneration is also higher [10]. Alternatively, balloon valvuloplasty, surgical valvotomy, or commissurotomy are the initial treatments of choice. If AVR is needed, the Ross procedure is recommended, in which aortic valve is replaced with patient’s own pulmonic valve, reducing the risk of patient-prosthesis mismatch [11, 12]. The autograft also has some capability to grow along with the patient’s heart. The Ross procedure is technically more challenging, with relatively high mortality, but is considered to be safe in experienced hands [10].
CONCLUSION
In Conclusion, unicuspid aortic valve is a rare congenital malformation that often leads to severe aortic stenosis. It is an important clinical entity that should be in the differential diagnosis of younger patients who present with symptoms of heart failure and with a systolic murmur that suggests aortic stenosis. Many cases are diagnosed peri-operatively. However, with evolving imaging technology, a higher incidence of preoperative diagnosis is expected. TEE has a relatively high specificity and sensitivity for diagnosis. Aortic valve repair with balloon valvuloplasty, surgical valvotomy, or commissurotomy are the initial treatments of choice in the young population and AVR is discouraged until patient is fully grown.
REFERENCES:
- Roberts W. C., Ko J. M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111(7):920–5.
- Mookadam F, Thota VR, Garcia-Lopez AM, Emani UR, Alharthi MS, Zamorano J, et al. Unicuspid aortic valve in adults: a systematic review. J Heart Valve Dis. 2010;19(1):79–85.
- Mookadam F, Thota VR, Lopez AM, Emani UR, Tajik AJ. Unicuspid aortic valve in children: a systematic review spanning four decades. J Heart Valve Dis. 2010;19(6):678–83.
- Falcone MW, Roberts WC, Morrow AG, Perloff JK. Congenital aortic stenosis resulting from a unicommissural valve. Clinical and anatomic features in twenty-one adult patients. Circulation. 1971;44(2):272–80.
- Kang S. D., Seol S. H., Park B. M., Kim D. K., Kim K. H., Kim D. I. et al. Incidental diagnosis of the unicuspid aortic valve with ascending aortic aneurysm in an asymptomatic adult. J Cardiovasc Ultrasound. 2011;19(2):102–4.
- Brantley H. P., Nekkanti R., Anderson C. A., Kypson A. P. Three-dimensional echocardiographic features of unicuspid aortic valve stenosis correlate with surgical findings. Echocardiography. 2012;29(8):E204–7.
- Edwards J. E. Pathologic aspects of cardiac valvular insufficiencies. AMA Arch Surg. 1958;77(4):634–49.
- Novaro G. M., Mishra M., Griffin B. P. Incidence and echocardiographic features of congenital unicuspid aortic valve in an adult population. J Heart Valve Dis. 2003;12(6):674–8.
- Chu JW, Picard MH, Agnihotri AK, Fitzsimons MG. Diagnosis of congenital unicuspid aortic valve in adult population: the value and limitation of transesophageal echocardiography. Echocardiography. 2010;27(9):1107–12.
- Alsoufi B. Aortic valve replacement in children: options and outcomes. J Saudi Heart Assoc. 2014;26(1):33–41.
- Sharabiani MT, Dorobantu DM, Mahani AS, Turner M, Peter Tometzki AJ, Angelini GD, et al. Aortic valve replacement and the Ross operation in children and young adults. J Am Coll Cardiol. 2016;67(24):2858–70.
- Svensson LG, Adams DH, Bonow RO, Kouchoukos NT, Miller DC. P.T. O'Gara, et al., Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg. 2013;95(6):S1–66.
- Brancaccio G, Polito A, Hoxha S, Gandolfo F, Giannico S, Amodeo A, et al. The Ross procedure in patients aged less than 18 years: the midterm results. J Thorac Cardiovasc Surg. 2014;147(1):383–8.
- Gibbs W. N., Hamman B. L., Roberts W. C., Schussler J. M. Diagnosis of congenital unicuspid aortic valve by 64-slice cardiac computed tomography. Proc (Bayl Univ Med Cent) 2008;21(2):139.
- Debl K., Djavidani B., Buchner S., Poschenrieder F., Heinicke N., Schmid C. et al. Unicuspid aortic valve disease: a magnetic resonance imaging study [in German] Rofo. 2008;180(11):983–7.
Authors:
Kulwant Bath, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Endocarditis of the Mitral Valve! And The Vicinity!!
Description
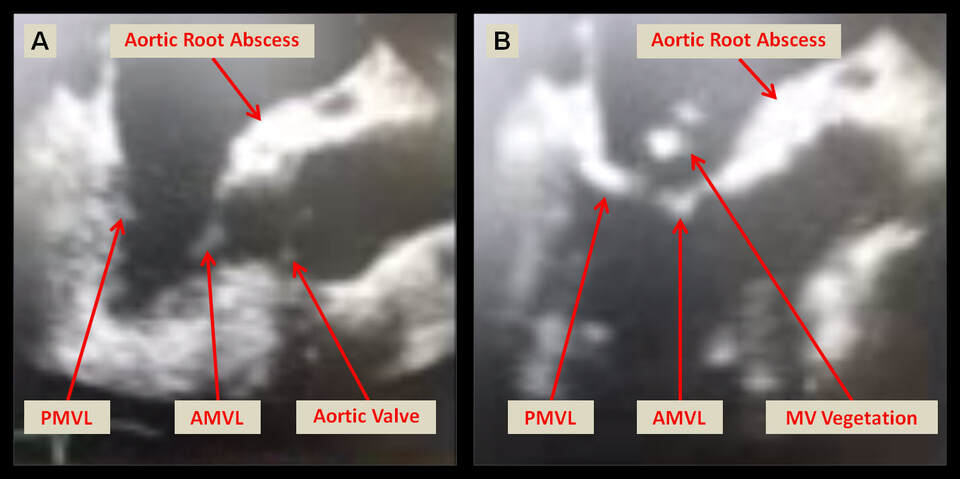
The above transesophageal echocardiography images from the mid-esophageal long axis view illustrate a diastolic frame (A) showing the anterior mitral valve leaflet (AMVL) and the posterior mitral valve leaflet (PMVL) in open position, while the aortic valve is in closed position. An aortic root thickening suspicious for abscess is noted. In the systolic frame (B) in addition to the native valvular structures, a mitral valve vegetation is visualized at the coaptation point of both mitral valve leaflets. The aortic root abscess is again visualized as labeled.
The aortic root is a complex structure and consists of the aortic annulus, the sinuses of Valsalva (or aortic sinuses), and the sinotubular junction. The internal structure of the aortic root consists of the aortic valve leaflets, the commissures, and the interleaflet triangles. It is located in close proximity to other cardiac structures, mainly the pulmonary valve anteriorly, the mitral valve to the left and posteriorly, and the tricuspid valve to the right and posteriorly, and, therefore, can be affected by pathology of such structures, including endocarditis or degenerative valve disease [1].
Echocardiography is widely used for the detection and follow-up of aortic root disease, due to its wide availability, relatively low cost, safety, and ability to assess hemodynamic parameters of the aortic valve [2]. Echocardiographic limitations include patient-specific factors (such as body habitus) and operator dependence. Three-dimensional echocardiography and transesophageal echocardiography can overcome some of these limitations, allowing for measurements in multiple planes [3].
Aortic root abscess should be suspected in patients with aortic valve endocarditis, especially those who fail to improve within 72 house on appropriate antibiotic therapy; especially in the presence of a prosthetic valve infection. Persistence or recrudescence of fever, persistently elevated white blood cell counts, other markers of systemic inflammation such as high C reactive protein, or the development of cutaneous manifestations or embolic phenomena while on adequate treatment all indicate uncontrolled infection. A lengthening PR interval on the surface ECG or development of heart block are particularly worrying features, and in the pre echocardiographic era determined the need for urgent surgery [4],
Aortic root abscess is a life threatening complication of both native and prosthetic valve infection, which requires coordinated and experienced management [5]. Once an aortic root abscess is detected, urgent surgery is required as antibiotics alone will fail to control the infection while surgery may be curative. There is increased morbidity and mortality when surgery is delayed [6].
References:
Authors:
Nikky Bardia, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Mansoor Mozayan, M.D., Ph.D.
Staff Cardiologist
MedStar Franklin Square Medical Center
Baltimore, MD
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The above transesophageal echocardiography images from the mid-esophageal long axis view illustrate a diastolic frame (A) showing the anterior mitral valve leaflet (AMVL) and the posterior mitral valve leaflet (PMVL) in open position, while the aortic valve is in closed position. An aortic root thickening suspicious for abscess is noted. In the systolic frame (B) in addition to the native valvular structures, a mitral valve vegetation is visualized at the coaptation point of both mitral valve leaflets. The aortic root abscess is again visualized as labeled.
The aortic root is a complex structure and consists of the aortic annulus, the sinuses of Valsalva (or aortic sinuses), and the sinotubular junction. The internal structure of the aortic root consists of the aortic valve leaflets, the commissures, and the interleaflet triangles. It is located in close proximity to other cardiac structures, mainly the pulmonary valve anteriorly, the mitral valve to the left and posteriorly, and the tricuspid valve to the right and posteriorly, and, therefore, can be affected by pathology of such structures, including endocarditis or degenerative valve disease [1].
Echocardiography is widely used for the detection and follow-up of aortic root disease, due to its wide availability, relatively low cost, safety, and ability to assess hemodynamic parameters of the aortic valve [2]. Echocardiographic limitations include patient-specific factors (such as body habitus) and operator dependence. Three-dimensional echocardiography and transesophageal echocardiography can overcome some of these limitations, allowing for measurements in multiple planes [3].
Aortic root abscess should be suspected in patients with aortic valve endocarditis, especially those who fail to improve within 72 house on appropriate antibiotic therapy; especially in the presence of a prosthetic valve infection. Persistence or recrudescence of fever, persistently elevated white blood cell counts, other markers of systemic inflammation such as high C reactive protein, or the development of cutaneous manifestations or embolic phenomena while on adequate treatment all indicate uncontrolled infection. A lengthening PR interval on the surface ECG or development of heart block are particularly worrying features, and in the pre echocardiographic era determined the need for urgent surgery [4],
Aortic root abscess is a life threatening complication of both native and prosthetic valve infection, which requires coordinated and experienced management [5]. Once an aortic root abscess is detected, urgent surgery is required as antibiotics alone will fail to control the infection while surgery may be curative. There is increased morbidity and mortality when surgery is delayed [6].
References:
- Berdajs DA. Aortic root morphology: a paradigm for successful reconstruction. Interact Cardiovasc Thorac Surg. 2016 Jan;22(1):85-91.
- Teichholz LE. Echocardiography in valvular heart disease. Prog Cardiovasc Dis. 1975 Jan-Feb;17(4):283-302.
- Michelena HI, Abel MD, Suri RM, et al. Intraoperative echocardiography in valvular heart disease: an evidence-based appraisal. Mayo Clin Proc. 2010 Jul;85(7):646-55.
- Seghatol F, Grinberg I. Left-sided endocarditis in intravenous drug users: a case report and review of the literature. Echocardiography. 2002 Aug;19(6):509-11.
- Chen GJ, Lo WC, Tseng HW, et al. Outcome of surgical intervention for aortic root abscess: a meta-analysis. Eur J Cardiothorac Surg. 2018 Apr 1;53(4):807-814.
- Perrotta S, Aljassim O, Jeppsson A, et al. Survival and quality of life after aortic root replacement with homografts in acute endocarditis. Ann Thorac Surg .200 Dec; 90(6):1862-7.
Authors:
Nikky Bardia, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Mansoor Mozayan, M.D., Ph.D.
Staff Cardiologist
MedStar Franklin Square Medical Center
Baltimore, MD
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Cleft Mitral! A Deleterious Primum Residue!
Description
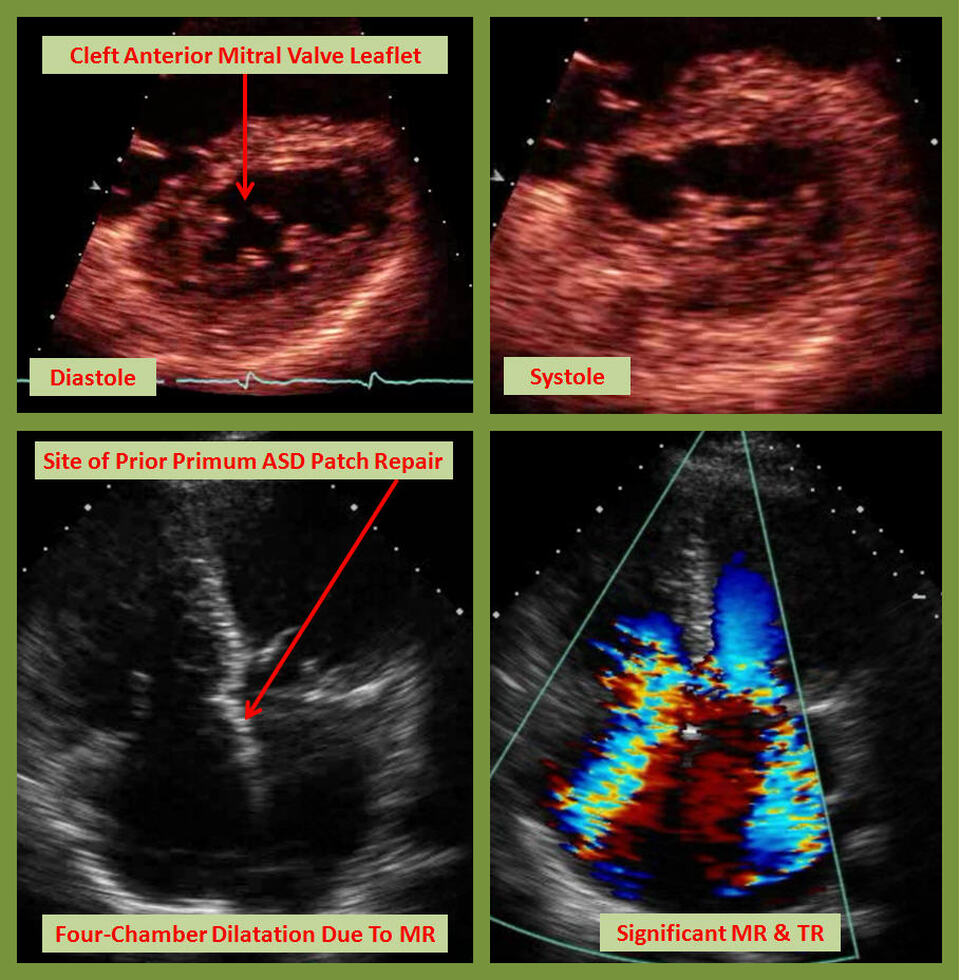
Atrial Septal Defect (ASD) is the most common congenital cardiac anomaly in the adult. The most common form is the ostium secundum, which is usually an isolated congenital anomaly, and often discovered incidentally. Sinus venosus ASD is associated with anomalous connection of the right upper pulmonary vein, while primum ASD is frequently associated with a cleft anterior mitral valve leaflet causing significant mitral regurgitation (MR). It is crucial to be aware of such associations in planning surgery, so that repair of these lesions is performed at the time of ASD closure [1].
Primum ASD (partial atrioventricular canal defect) can be accompanied by a variety of anomalies of the endocardial cushion, most prominently a cleft mitral and/or tricuspid valve leaflet. It can also be associated with left-sided obstructive lesions such as coarctation, subaortic stenosis, a single papillary muscle (parachute mitral valve causing congenital mitral stenosis) or double orifice left ventricle, and can present with significant CHF symptoms if not detected and repaired early. These findings may be seen in up to 10% of patients with primum ASD [2].
Short- and long-term survival for repair of isolated primum ASD approaches 100% with need for reintervention, typically directed at management of MR, in only 5 to 10%. Complete repair involving closure of the primum atrial septal defect and repair of the cleft of the mitral valve with pericardial patch has been the practice since 1982 [3].
Patients, who do not undergo closure of the cleft, can have significant mitral valve regurgitation later in life. The postoperative grade of MR is an independent risk factor for late significant MR, so even mild regurgitation postoperatively should be addressed. While late development of moderate-to-severe MR can either be repaired or replaced, the outcomes are better with early repair. A challenging technical characteristic for the surgeon is the ability to maintain the mitral valve competency in the postoperative period [4].
Therefore, a high index of suspicion should be maintained in the work-up and planning for surgery in atrial septal defects, so that associated lesions can be detected with the use of echocardiography, CT angiography and cardiac MRI, in addition to identifying a skilled surgeon capable of performing such complex repairs [5].
References:
Authors:
Nikky Bardia, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Amod Amritphale, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Atrial Septal Defect (ASD) is the most common congenital cardiac anomaly in the adult. The most common form is the ostium secundum, which is usually an isolated congenital anomaly, and often discovered incidentally. Sinus venosus ASD is associated with anomalous connection of the right upper pulmonary vein, while primum ASD is frequently associated with a cleft anterior mitral valve leaflet causing significant mitral regurgitation (MR). It is crucial to be aware of such associations in planning surgery, so that repair of these lesions is performed at the time of ASD closure [1].
Primum ASD (partial atrioventricular canal defect) can be accompanied by a variety of anomalies of the endocardial cushion, most prominently a cleft mitral and/or tricuspid valve leaflet. It can also be associated with left-sided obstructive lesions such as coarctation, subaortic stenosis, a single papillary muscle (parachute mitral valve causing congenital mitral stenosis) or double orifice left ventricle, and can present with significant CHF symptoms if not detected and repaired early. These findings may be seen in up to 10% of patients with primum ASD [2].
Short- and long-term survival for repair of isolated primum ASD approaches 100% with need for reintervention, typically directed at management of MR, in only 5 to 10%. Complete repair involving closure of the primum atrial septal defect and repair of the cleft of the mitral valve with pericardial patch has been the practice since 1982 [3].
Patients, who do not undergo closure of the cleft, can have significant mitral valve regurgitation later in life. The postoperative grade of MR is an independent risk factor for late significant MR, so even mild regurgitation postoperatively should be addressed. While late development of moderate-to-severe MR can either be repaired or replaced, the outcomes are better with early repair. A challenging technical characteristic for the surgeon is the ability to maintain the mitral valve competency in the postoperative period [4].
Therefore, a high index of suspicion should be maintained in the work-up and planning for surgery in atrial septal defects, so that associated lesions can be detected with the use of echocardiography, CT angiography and cardiac MRI, in addition to identifying a skilled surgeon capable of performing such complex repairs [5].
References:
- Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet. 2014 May 31;383(9932):1921-32.
- Sadeghi AM, Laks H, Pearl JM. Primum atrial septal defect. Semin Thorac Cardiovasc Surg. 1997 Jan;9(1):2-7.
- Jemielity M, Perek B, Paluszkiewicz L, et al. Results of surgical repair of ostium primum atrial septal defect in adult patients. J Heart Valve Dis. 2001 Jul;10(4):525-9.
- Kharouf R, Luxenberg DM, Khalid O, et al. Atrial septal defect: spectrum of care. Pediatr Cardiol. 2008 Mar;29(2):271-80.
- Rigatelli G, Cardaioli P, Hijazi ZM. Contemporary clinical management of atrial septal defects in the adult. Expert Rev Cardiovasc Ther. 2007 Nov;5(6):1135-46.
Authors:
Nikky Bardia, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Farnoosh Rahimi, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Amod Amritphale, M.D.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL