June 2021 Issue
ISSN 2689-291X
ISSN 2689-291X
Peripartum Cardiomyopathy..
A Mysterious Misfortune of Pregnancy!
Description
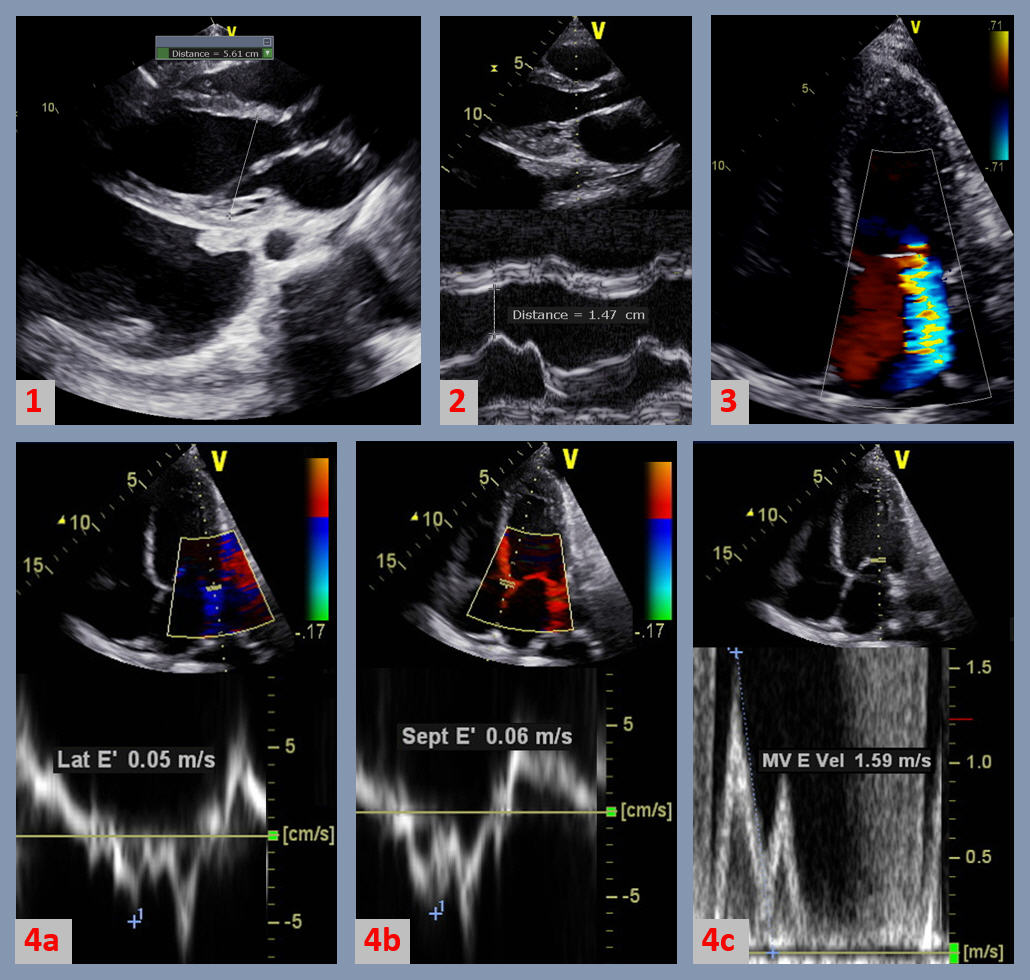
The figure above demonstrated some of the echocardiographic features seen in peripartum cardiomyopathy (PPCM), a type of dilated cardiomyopathy (DCM). Figure 1 is a 2-dimensional (2D) parasternal long axis view of the left ventricle (LV) showing a dilated LV > 5.2 cm, and bilateral pleural effusions. Figure 2 has an M-mode insert showing an increased E-point to septal separation of greater than 0.6 cm, indicative of LV dilatation and systolic dysfunction. Figure 3 is a 2D apical 4-chamber view with color flow Doppler showing moderate secondary mitral regurgitation due to dilatation of the mitral valve annulus. Figure 4 demonstrates the diastolic properties in DCM and PPCM. There is impaired LV diastolic relaxation shown by a decrease in the mitral annular diastolic tissue Doppler velocities of < 0.10 m/s in the lateral annulus (Figure 4a) and < 0.07 m/s in the septal velocities (Figure 4b). The mitral valve diastolic spectral Doppler flow velocities (Figure 4c) reveal elevated E wave velocity, with and E/A ratio of > 0.8 and an average E/e’ of > 14, indicative of grade II diastolic dysfunction and elevated left atrial pressure: signs of volume overload in congestive heart failure (CHF).
Discussion
Peripartum cardiomyopathy (PPCM) is a rare form of DCM of uncertain etiology which usually presents late in pregnancy or, more commonly, up to 5 months postpartum [1]. PPCM is more common in females > 30 years of age, and in those with prior hypertension and preeclampsia [2]. Worldwide, the prevalence of PPCM is quite variable with a reported highest incidence of approximately 1% of deliveries in Nigeria, and lowest incidence of about 0.006% in Japan [3]. About 1,000 to 1,300 women develop the condition in the U.S. each year, with an incidence of approximately 0.05% of deliveries [4].
Several predisposing factors for PPCM have been reported including but not limited to pre-eclampsia, hypertension, advanced maternal age, and multiple gestation pregnancy [5]. PPCM tends to be more prevalent in African American females [6], who have also been reported to have a poorer prognosis and more delayed recovery despite treatment [7]. Familial forms of PPCM, similar to dilated cardiomyopathies in general, have been described [8].
Symptoms of early heart failure due to PPCM are often difficult to distinguish from the usual symptoms of dyspnea and fatigue seen in late pregnancy, underscoring the importance of early investigation of signs of fluid overload [9]. Frequently encountered symptoms include shortness of breath, orthopnea, palpitations and nocturia, all typical symptoms of congestive heart failure and volume overload.
Diagnosis of PPCM is made when the following criteria are met: 1. HF within the specific time frame (last month of pregnancy until 5 months postpartum), 2. Left ventricular ejection fraction <45%, 3. No other cause of reduced EF can be found [10]. The etiology and pathophysiology of PPCM remains obscure and likely multifactorial. Proposed mechanisms include vascular inflammation, abnormal immune response, hormonal derangements, excess catecholamines [11]. Genetic resemblance to dilated cardiomyopathy has also been described which may offer hope for common diagnostic tools, therapies and counseling [12].
Management of peripartum cardiomyopathy is largely limited to symptom relief with diuretics in addition to the same neurohormonal antagonists used in other forms of cardiomyopathy, avoiding angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) during pregnancy and lactation. Greater than 50% pf patient will recover, however, some patients may decompensate requiring inotropic support, ventricular assist devices (VAD), extracorporeal membrane oxygenation (ECMO), and heart transplantation which carries a poorer prognosis compared with transplants in non-PPCM heart failure patients [13]. Despite initial enthusiasm about the potential role of bromocriptine in suppressing pituitary release of prolactin, this has not consistently altered clinical outcomes, and currently and no proven disease-specific therapies exist in the US [14]. The importance of medication compliance and lifestyle modification should be emphasized with each patient. In addition, it is recommended that women avoid any future pregnancies due to the appreciable recurrence of heart failure, especially when the LV systolic function remains impaired [15]. In summary, PPCM is a mysterious misfortune of pregnancy which can benefit from a multi-specialty interdisciplinary team approach addressing the physical and mental health of patients, with proper risk stratification and counseling to optimize outcome in these young patients and avoid tragic consequences.
References:
Authors:
Sarah Dabbas, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Siva Chiranjeevi, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The figure above demonstrated some of the echocardiographic features seen in peripartum cardiomyopathy (PPCM), a type of dilated cardiomyopathy (DCM). Figure 1 is a 2-dimensional (2D) parasternal long axis view of the left ventricle (LV) showing a dilated LV > 5.2 cm, and bilateral pleural effusions. Figure 2 has an M-mode insert showing an increased E-point to septal separation of greater than 0.6 cm, indicative of LV dilatation and systolic dysfunction. Figure 3 is a 2D apical 4-chamber view with color flow Doppler showing moderate secondary mitral regurgitation due to dilatation of the mitral valve annulus. Figure 4 demonstrates the diastolic properties in DCM and PPCM. There is impaired LV diastolic relaxation shown by a decrease in the mitral annular diastolic tissue Doppler velocities of < 0.10 m/s in the lateral annulus (Figure 4a) and < 0.07 m/s in the septal velocities (Figure 4b). The mitral valve diastolic spectral Doppler flow velocities (Figure 4c) reveal elevated E wave velocity, with and E/A ratio of > 0.8 and an average E/e’ of > 14, indicative of grade II diastolic dysfunction and elevated left atrial pressure: signs of volume overload in congestive heart failure (CHF).
Discussion
Peripartum cardiomyopathy (PPCM) is a rare form of DCM of uncertain etiology which usually presents late in pregnancy or, more commonly, up to 5 months postpartum [1]. PPCM is more common in females > 30 years of age, and in those with prior hypertension and preeclampsia [2]. Worldwide, the prevalence of PPCM is quite variable with a reported highest incidence of approximately 1% of deliveries in Nigeria, and lowest incidence of about 0.006% in Japan [3]. About 1,000 to 1,300 women develop the condition in the U.S. each year, with an incidence of approximately 0.05% of deliveries [4].
Several predisposing factors for PPCM have been reported including but not limited to pre-eclampsia, hypertension, advanced maternal age, and multiple gestation pregnancy [5]. PPCM tends to be more prevalent in African American females [6], who have also been reported to have a poorer prognosis and more delayed recovery despite treatment [7]. Familial forms of PPCM, similar to dilated cardiomyopathies in general, have been described [8].
Symptoms of early heart failure due to PPCM are often difficult to distinguish from the usual symptoms of dyspnea and fatigue seen in late pregnancy, underscoring the importance of early investigation of signs of fluid overload [9]. Frequently encountered symptoms include shortness of breath, orthopnea, palpitations and nocturia, all typical symptoms of congestive heart failure and volume overload.
Diagnosis of PPCM is made when the following criteria are met: 1. HF within the specific time frame (last month of pregnancy until 5 months postpartum), 2. Left ventricular ejection fraction <45%, 3. No other cause of reduced EF can be found [10]. The etiology and pathophysiology of PPCM remains obscure and likely multifactorial. Proposed mechanisms include vascular inflammation, abnormal immune response, hormonal derangements, excess catecholamines [11]. Genetic resemblance to dilated cardiomyopathy has also been described which may offer hope for common diagnostic tools, therapies and counseling [12].
Management of peripartum cardiomyopathy is largely limited to symptom relief with diuretics in addition to the same neurohormonal antagonists used in other forms of cardiomyopathy, avoiding angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) during pregnancy and lactation. Greater than 50% pf patient will recover, however, some patients may decompensate requiring inotropic support, ventricular assist devices (VAD), extracorporeal membrane oxygenation (ECMO), and heart transplantation which carries a poorer prognosis compared with transplants in non-PPCM heart failure patients [13]. Despite initial enthusiasm about the potential role of bromocriptine in suppressing pituitary release of prolactin, this has not consistently altered clinical outcomes, and currently and no proven disease-specific therapies exist in the US [14]. The importance of medication compliance and lifestyle modification should be emphasized with each patient. In addition, it is recommended that women avoid any future pregnancies due to the appreciable recurrence of heart failure, especially when the LV systolic function remains impaired [15]. In summary, PPCM is a mysterious misfortune of pregnancy which can benefit from a multi-specialty interdisciplinary team approach addressing the physical and mental health of patients, with proper risk stratification and counseling to optimize outcome in these young patients and avoid tragic consequences.
References:
- Garg J, Palaniswamy C, Lanier GM. Peripartum cardiomyopathy: definition, incidence, etiopathogenesis, diagnosis, and management. Cardiol Rev. 2015 Mar-Apr;23(2):69-78.
- Elkayam U, Jalnapurkar S, Barakat M. Peripartum cardiomyopathy. Cardiol Clin. 2012 Aug;30(3):435-40.
- Isogai T, Kamiya CA. Worldwide Incidence of Peripartum Cardiomyopathy and Overall Maternal Mortality. Int Heart J. 2019 May 30;60(3):503-511.
- Davis MB, Arany Z, McNamara DM, et al. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020 Jan 21;75(2):207-221.
- Shani H, Kuperstein R, Berlin A, et al. Peripartum cardiomyopathy – risk factors, characteristics and long-term follow-up. J Perinat Med. 2015 Jan;43(1):95-101.
- Sinkey RG, Rajapreyar IN, Szychowski JM, et al. Racial disparities in peripartum cardiomyopathy: eighteen years of observations. J Matern Fetal Neonatal Med. 2020 Jun 7:1-8.
- Irizarry OC, Levine LD, Lewey J, et al. Comparison of Clinical Characteristics and Outcomes of Peripartum Cardiomyopathy Between African American and Non-African American Women. JAMA Cardiol. 2017 Nov 1;2(11):1256-1260.
- van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010 May 25;121(20):2169-75.
- Lewey J, Levine LD, Elovitz MA, et al. Importance of Early Diagnosis in Peripartum Cardiomyopathy. Hypertension. 2020 Jan;75(1):91-97.
- Bauersachs J, Koenig T. Devil in Disguise: Hints and Pitfalls in Diagnosis of Peripartum Cardiomyopathy. Circ Heart Fail. 2018 Apr;11(4):e004620.
- Ricke-Hoch M, Pfeffer TJ, Hilfiker-Kleiner D. Peripartum cardiomyopathy: basic mechanisms and hope for new therapies. Cardiovasc Res. 2020 Mar 1;116(3):520-531.
- Goli R, Li J, Brandimarto J, et al; IMAC-2 and IPAC Investigators, Arany Z. Genetic and Phenotypic Landscape of Peripartum Cardiomyopathy. Circulation. 2021 May 11;143(19):1852-1862.
- Robbins KS, Krause M, Nguyen AP, et al. Peripartum Cardiomyopathy: Current Options for Treatment and Cardiovascular Support. J Cardiothorac Vasc Anesth. 2019 Oct;33(10):2814-2825.
- Goland S, Elkayam U. Peripartum cardiomyopathy: approach to management. Curr Opin Cardiol. 2018 May;33(3):347-353.
- Bauersachs J, König T, van der Meer P, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019 Jul;21(7):827-843.
Authors:
Sarah Dabbas, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Siva Chiranjeevi, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Christopher Malozzi, D.O.
Assistant Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL