May 2019 Issue
ISSN 2689-291X
ISSN 2689-291X
Challenging Images
Acute Severe Aortic Regurgitation..Reverse Circulation!
Description
Acute severe aortic regurgitation may result from acute aortic dissection, acute endocarditis, or less commonly, trauma [1]. The sudden massive increase in both preload and afterload overwhelm the left ventricle’s compensatory mechanisms, resulting in abrupt rise in the left ventricular end diastolic pressure and acute congestive heart failure [2].
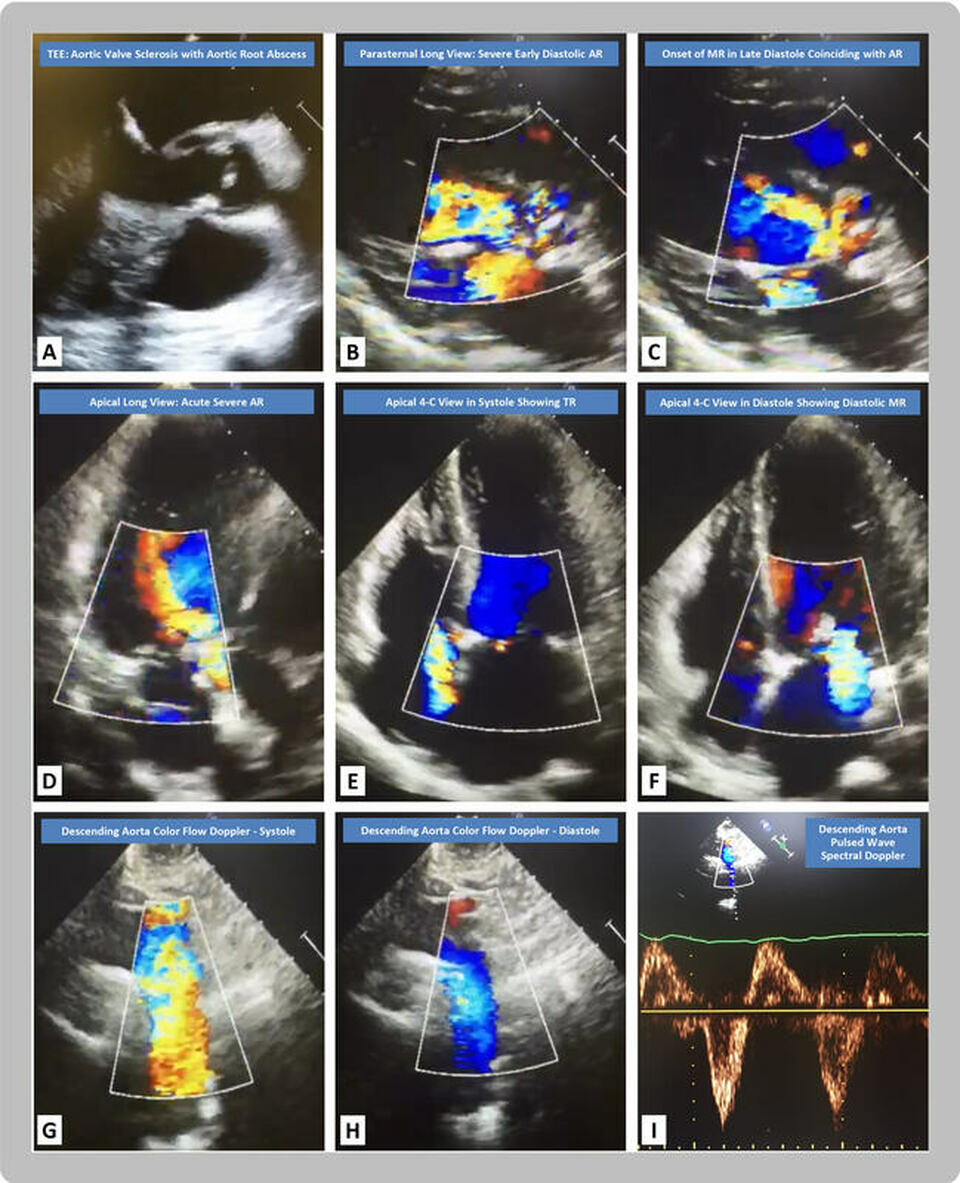
In the figure, the transesophageal echocardiography (TEE) midesophageal long axis view (A, video 1) reveals calcified aortic cusps with an anterior aortic root echolucent space consistent with an abscess. The 2-D parasternal long axis view in early diastole (B, video 2) shows severe aortic regurgitation (AR) by color-flow Doppler, while the late diastolic frame (C) reveals a jet of diastolic mitral regurgitation (MR) concurrent with the AR jet. The 2-D apical long axis view (D) demonstrates the early diastolic jet of severe AR by color-flow Doppler. The apical four-chamber (4-C) views reveal tricuspid regurgitation (TR) in systole (E) and MR in late diastole (F). The suprasternal notch 2-D and color flow Doppler views display normal antegrade flow in systole within the descending aorta (G), with reversal of flow in diastole (H). The pulsed wave spectral Doppler of the descending aorta (I) reveals an abnormal holodiastolic flow reversal within the descending aorta.
Flow reversal within the descending aorta is a well described sign of significant aortic regurgitation, especially when the reversal is holodiastolic [3]. The increasing pressure in the left ventricle throughout diastole results in higher left ventricular end diastolic pressure than left atrial pressure. This causes presystolic reversal of flow and late diastolic MR [4]. Another mechanism for the diastolic MR is spread of the aortic valve infection to the mitral valve causing leaflet perforation resulting in flow reversal through the perforation once the left ventricular pressure exceeds the left atrial pressure in late diastole [5].
References:
Authors:
Keerthana Karumbaiah, M.D.
Staff Cardiologist
Shannon Clinic
San Angelo, TX
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Acute severe aortic regurgitation may result from acute aortic dissection, acute endocarditis, or less commonly, trauma [1]. The sudden massive increase in both preload and afterload overwhelm the left ventricle’s compensatory mechanisms, resulting in abrupt rise in the left ventricular end diastolic pressure and acute congestive heart failure [2].
In the figure, the transesophageal echocardiography (TEE) midesophageal long axis view (A, video 1) reveals calcified aortic cusps with an anterior aortic root echolucent space consistent with an abscess. The 2-D parasternal long axis view in early diastole (B, video 2) shows severe aortic regurgitation (AR) by color-flow Doppler, while the late diastolic frame (C) reveals a jet of diastolic mitral regurgitation (MR) concurrent with the AR jet. The 2-D apical long axis view (D) demonstrates the early diastolic jet of severe AR by color-flow Doppler. The apical four-chamber (4-C) views reveal tricuspid regurgitation (TR) in systole (E) and MR in late diastole (F). The suprasternal notch 2-D and color flow Doppler views display normal antegrade flow in systole within the descending aorta (G), with reversal of flow in diastole (H). The pulsed wave spectral Doppler of the descending aorta (I) reveals an abnormal holodiastolic flow reversal within the descending aorta.
Flow reversal within the descending aorta is a well described sign of significant aortic regurgitation, especially when the reversal is holodiastolic [3]. The increasing pressure in the left ventricle throughout diastole results in higher left ventricular end diastolic pressure than left atrial pressure. This causes presystolic reversal of flow and late diastolic MR [4]. Another mechanism for the diastolic MR is spread of the aortic valve infection to the mitral valve causing leaflet perforation resulting in flow reversal through the perforation once the left ventricular pressure exceeds the left atrial pressure in late diastole [5].
References:
- Maheshwari V, Barr B, Srivastava M. Acute Valvular Heart Disease. Cardiol Clin. 2018 Feb;36(1):115-127.
- Bugan B, Yildirim E, Celik M, et al. Acute Aortic Regurgitation in the Current Era of Percutaneous Treatment: Pathophysiology and Hemodynamics. J Heart Valve Dis. 2017 Jan;26(1):22-31.
- Sutton DC, Kluger R, Ahmed SU, et al. Flow reversal in the descending aorta: a guide to intraoperative assessment of aortic regurgitation with transesophageal echocardiography. J Thorac Cardiovasc Surg. 1994 Sep;108(3):576-82.
- Downes TR, Nomeir AM, Hackshaw BT, et al. Diastolic mitral regurgitation in acute but not chronic aortic regurgitation: implications regarding the mechanism of mitral closure. Am Heart J. 1989 May;117(5):1106-12.
- Konka M, Kusmierczyk-Droszcz B, Wozniak O, et al. Aortic regurgitation and unusual diastolic mitral regurgitation. Eur J Echocardiogr. 2008 Sep;9(5):709-11.
Authors:
Keerthana Karumbaiah, M.D.
Staff Cardiologist
Shannon Clinic
San Angelo, TX
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Lacosamide-Indcued Brugada! & SA Exit Block!!
Description
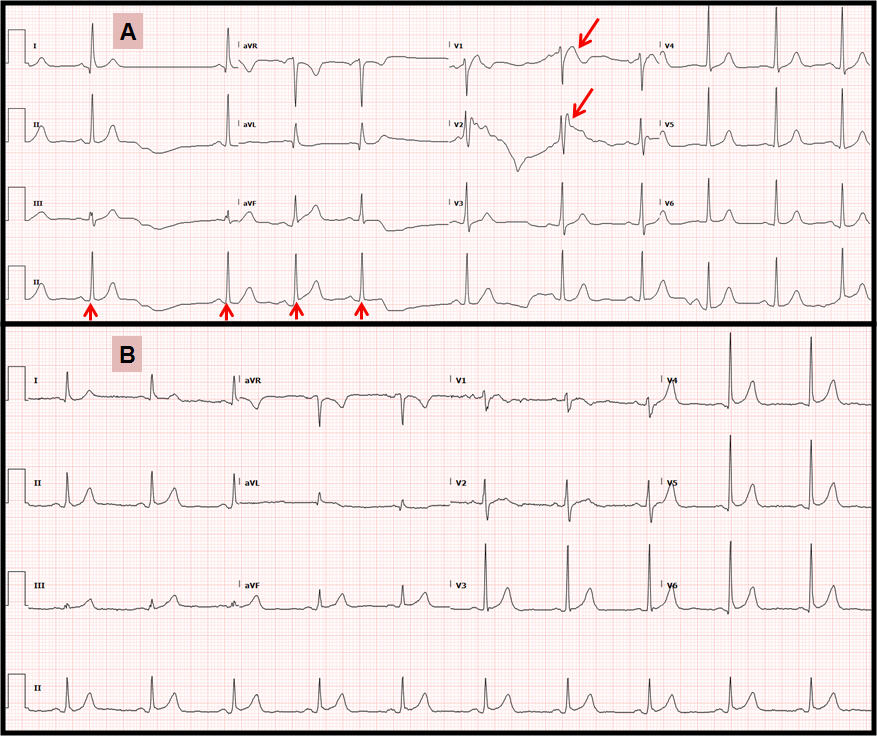
The electrocardiogram shown above reveals Brugada type I pattern in V1 & V2 (large arrows), and sinoatrial exit block (small arrows) while on Lacosamide (A). Changes resolved after stopping Lacosamide (B).
Sodium channel blockers have been used in the treatment of focal and generalized tonic-clonic seizures for more than 70 years [1]. SCB stabilize hyper-excitable neuronal membranes by selectively increasing the slow inactivation of voltage-gated sodium channels [2]. These drugs reduce the action potential duration and the maximal upstroke velocity (Vmax) in the isolated canine Purkinje fibers, causing unwanted side effects [3].
Lacosamide, carbamazepine, and lamotrigine all inhibit the cardiac sodium channel SCN5A in a concentration-dependent manner [6]. Disruption of the SCN5A channel causes severe arrhythmia, including ventricular tachycardia, in patients with Brugada syndrome, a channelopathy which is associated with an increased risk of ventricular tachycardia and sudden death [6].
Provocation with a sodium channel blocker can unmask latent Brugada syndrome. Brugda syndrome causes arrhythmia in the His‐Purkinje system, the right ventricle, the sinus node and atrium, due to ion channel mutations. Sinus node dysfunction may therefore be another cause of syncope in Brugada syndrome [7].
Electrocardiographically characterized by a distinct ST-segment elevation in the right precordial leads, the Brugada syndrome is associated with a high risk for sudden cardiac death in young and otherwise healthy adults. The ECG manifestations of Brugada syndrome are often dynamic or concealed and may be unmasked or modulated by sodium channel blockers [8].
Sudden unexpected death in epilepsy (SUDEP) is the most common epilepsy-related cause of death [9]. While the precise pathophysiological mechanisms underlying SUDEP are still uncertain. The potential role of antiepileptic drugs has been suggested [9].
Drugs which decrease inward currents (fast sodium current) at the end of phase 1 of the action potential can accentuate or unmask ST-segment elevation, similar to that found in the Brugada syndrome, thus producing acquired forms of the Brugada syndrome [10,11]. IV use of phenytoin causes reluctance to its usage due to possible cardiovascular effects. However, there is no reported cardiotoxicity resulting from oral overdose of phenytoin. One patient with post-traumatic epilepsy who received oral phenytoin for five months and developed life-threatening junctional bradycardia, with his serum phenytoin level reaching up to 91 microg/mL. Severe phenytoin overdose should be considered in any patient with dysrhythmia and cardiovascular collapse on oral phenytoin [12].
A case of complete atrioventricular block with ventricular asystole was also reported in a patient receiving intravenous phenytoin [13].
Carbamazepine was suspected of inducing sinus node dysfunction and atrioventricular block. After few months of carbamazepine therapy, patients were reportedly treated in emergency rooms for bradyarrhythmia [14].
A study of the effects of lamotrigine on ECG intervals in healthy volunteer population indicated that the PR interval may be slightly prolonged, especially at high doses of lamotrigine [15].
Lacosamide is a relatively new antiseizure agent approved in the United States and Europe for adjuvant treatment of partial-onset seizures [16], however, experiments examining the mechanism of lacosamide block of neuronal sodium channels have suggested that the interaction of lacosamide with sodium channels is fundamentally different from that of the classic antiepileptic drugs in that lacosamide appears to selectively bind to the slow-inactivated state of the channel [16] suggesting a different binding site and novel mode of action.
Clinical studies have shown a small, dose-related increase in PR interval associated with lacosamide use [17]. There are several case reports which reported cardiac conduction abnormalities in patients with epilepsy while on lacosmaide. A case of second‐degree atrioventricular block was reported to be caused by the addition of lacosamide to other antiepilepsy medications known to prolong the PR interval, resulting in hypotension and bradycardia [18].
One patient was reported who experienced ventricular tachycardia during a cardiac stress test [6]. More reported cases in patients taking lacosamide at a higher than approved dosage: one experienced atrial flutter/fibrillation ( on 600 mg/day) [19], while the other with low dose had atrial fibrillation [20].
One case was in a child with subclinical seizures, hypoplastic left-heart syndrome, and previously well-controlled atrial tachycardia, who experienced atrial tachycardia coinciding with lacosamide treatment [21]. All patients completely recovered after discontinuing lacosamide.
References:
Authors:
Kulwant Bath, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Arshia Akbar, M.D.
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
The electrocardiogram shown above reveals Brugada type I pattern in V1 & V2 (large arrows), and sinoatrial exit block (small arrows) while on Lacosamide (A). Changes resolved after stopping Lacosamide (B).
Sodium channel blockers have been used in the treatment of focal and generalized tonic-clonic seizures for more than 70 years [1]. SCB stabilize hyper-excitable neuronal membranes by selectively increasing the slow inactivation of voltage-gated sodium channels [2]. These drugs reduce the action potential duration and the maximal upstroke velocity (Vmax) in the isolated canine Purkinje fibers, causing unwanted side effects [3].
Lacosamide, carbamazepine, and lamotrigine all inhibit the cardiac sodium channel SCN5A in a concentration-dependent manner [6]. Disruption of the SCN5A channel causes severe arrhythmia, including ventricular tachycardia, in patients with Brugada syndrome, a channelopathy which is associated with an increased risk of ventricular tachycardia and sudden death [6].
Provocation with a sodium channel blocker can unmask latent Brugada syndrome. Brugda syndrome causes arrhythmia in the His‐Purkinje system, the right ventricle, the sinus node and atrium, due to ion channel mutations. Sinus node dysfunction may therefore be another cause of syncope in Brugada syndrome [7].
Electrocardiographically characterized by a distinct ST-segment elevation in the right precordial leads, the Brugada syndrome is associated with a high risk for sudden cardiac death in young and otherwise healthy adults. The ECG manifestations of Brugada syndrome are often dynamic or concealed and may be unmasked or modulated by sodium channel blockers [8].
Sudden unexpected death in epilepsy (SUDEP) is the most common epilepsy-related cause of death [9]. While the precise pathophysiological mechanisms underlying SUDEP are still uncertain. The potential role of antiepileptic drugs has been suggested [9].
Drugs which decrease inward currents (fast sodium current) at the end of phase 1 of the action potential can accentuate or unmask ST-segment elevation, similar to that found in the Brugada syndrome, thus producing acquired forms of the Brugada syndrome [10,11]. IV use of phenytoin causes reluctance to its usage due to possible cardiovascular effects. However, there is no reported cardiotoxicity resulting from oral overdose of phenytoin. One patient with post-traumatic epilepsy who received oral phenytoin for five months and developed life-threatening junctional bradycardia, with his serum phenytoin level reaching up to 91 microg/mL. Severe phenytoin overdose should be considered in any patient with dysrhythmia and cardiovascular collapse on oral phenytoin [12].
A case of complete atrioventricular block with ventricular asystole was also reported in a patient receiving intravenous phenytoin [13].
Carbamazepine was suspected of inducing sinus node dysfunction and atrioventricular block. After few months of carbamazepine therapy, patients were reportedly treated in emergency rooms for bradyarrhythmia [14].
A study of the effects of lamotrigine on ECG intervals in healthy volunteer population indicated that the PR interval may be slightly prolonged, especially at high doses of lamotrigine [15].
Lacosamide is a relatively new antiseizure agent approved in the United States and Europe for adjuvant treatment of partial-onset seizures [16], however, experiments examining the mechanism of lacosamide block of neuronal sodium channels have suggested that the interaction of lacosamide with sodium channels is fundamentally different from that of the classic antiepileptic drugs in that lacosamide appears to selectively bind to the slow-inactivated state of the channel [16] suggesting a different binding site and novel mode of action.
Clinical studies have shown a small, dose-related increase in PR interval associated with lacosamide use [17]. There are several case reports which reported cardiac conduction abnormalities in patients with epilepsy while on lacosmaide. A case of second‐degree atrioventricular block was reported to be caused by the addition of lacosamide to other antiepilepsy medications known to prolong the PR interval, resulting in hypotension and bradycardia [18].
One patient was reported who experienced ventricular tachycardia during a cardiac stress test [6]. More reported cases in patients taking lacosamide at a higher than approved dosage: one experienced atrial flutter/fibrillation ( on 600 mg/day) [19], while the other with low dose had atrial fibrillation [20].
One case was in a child with subclinical seizures, hypoplastic left-heart syndrome, and previously well-controlled atrial tachycardia, who experienced atrial tachycardia coinciding with lacosamide treatment [21]. All patients completely recovered after discontinuing lacosamide.
References:
- Giulia Curia, Giuseppe Biagini, Emilio Perucca, and Massimo Avoli Lacosamide-A New Approach to Target Voltage-Gated Sodium Currents in Epileptic Disorders, 2009; 23(7): 555–568.
- Pamela Doty, David Hebert, Francois-Xavier Mathy, William Byrnes, James Zackheim, and Kelly Simontacchi. Development of lacosamide for the treatment of partial-onset seizures2013 Jul; 1291(1): 56–68.
- J. Thomas Bigger Jr. and William J. Mandel Effect of lidocaine on the electrophysiological properties of ventricular muscle and Purkinje fibers Vol 49 1970;49(1) 63-77.
- Ajith Cherian and Sanjeev V. Thomas1 Status epilepticus 2009 Jul-Sep; 12(3): 140–153.
- Brodie MJ. Sodium Channel Blockers in the Treatment of Epilepsy.Pub med Jul; 31(7):527-534.
- Andrew C.DeGiorgio Tamara E.Desso LanceLee Christopher M.DeGiorgio Ventricular tachycardia associated with lacosamide co-medication in drug-resistant epilepsy Volume 1, 2013, Pages 26-28.
- Hayashi H, Sumiyoshi M, Nakazato Y, Dada H. Brugada syndrome and sinus node dysfunction. J Arrhythmia. 2018; 34:216–221).
- Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, Shimizu W, Schulze-Bahr E, Tan H, Wilde A Brugada syndrome: report of the second consensus conference.2005 Apr;2(4):429-40.)
- QTc prolongation by antiepileptic drugs and the risk of torsade de pointes in patients with epilepsy Ashley E. Feldman Email the author Ashley E. Feldman Barry E. Gidal Correspondence information about the author Barry E. Gilda March 2013Volume 26, Issue3, Pages 421–4
- J Electrocardiol. Shimizu W Acquired forms of the Brugada syndrome. 2005 Oct;38(4 Suppl):22-5.
- Brugada syndrome Johnson Francis, and Charles Antzelevitch, 2005 may 25:101 (2): 173-178
- Su CM, Kung CT, Wang YC, Lu CH, Life-threatening cardiotoxicity due to chronic oral phenytoin overdose 2009 Mar-Apr;57(2):200-2
- Randazzo DN, Ciccone A, Schweitzer P, Winters SLComplete atrioventricular block with ventricular asystole following infusion of intravenous phenytoin.1995 Apr;28(2):157-9)
- Kan T, Itaru H, Jun-ichirou W. Carbamazepine-induced Sinus Node Dysfunction and Atrioventricular Block in Elderly Women1998 Volume 39 Issue 4 Pages 469-479
- Ruth Dixon, Sarah Alexander, Neil Brickel. Effect of lamotrigine on the PR interval in healthy subjects, 17th November 2010.
- Sooyeon Jo and Bruce P. Bean Lacosamide Inhibition of Nav1.7 Voltage-Gated Sodium Channels: Slow Binding to Fast-Inactivated States. Published online 2017 Apr. 91(4): 277–286
- Jonathan J Halford, MD and Marc Lapointe Clinical Perspectives on Lacosamide 2009 Jan; 9(1): 1–9.
- Ahmad Nizam Krishna Mylavarapu Dwithiya Thomas Klara Briskin Brenda Wu Deepak Saluja Stephen Wong Lacosamide‐induced second‐degree atrioventricular block in a patient with partial epilepsyFirst published: 29 July 2011).
- Christopher M. DeGiorgioAtrial flutter/atrial fibrillation associated with lacosamide for partial seizures July 2010Volume 18, Issue 3, Pages 322–324).
- Kenneth R.Kaufman Arnaldo E.Velez Low-dose lacosamide-induced atrial fibrillation: Case analysis with literature review.
- Loomba, R.S., Singh, A.K., Kovach, J., and Gudausky, T.M. Lacosamide-induced atrial tachycardia in a child with hypoplastic left-heart syndrome: the importance of assessing additional proarrhythmic risks. Cardiol Young. 2015; 25: 806–809.
Authors:
Kulwant Bath, M.D.
Internal Medicine Resident
University of South Alabama
Mobile, AL
Arshia Akbar, M.D.
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Rafique, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL