September 2018 Issue
ISSN 2689-291X
ISSN 2689-291X
Historical Perspective
Angioplasty Beginnings..40 Years Later
Roots of cardiac catheterization can be traced back as far as the 18th century, when brass pipes were used to measure pressures in a horse’s heart. Over the next 200 years, coronary angiography evolved greatly, making it possible to image the coronary arteries in humans [1]. Angiography gave rise to angioplasty inadvertently in the 1960s, providing the stepping-stone for therapeutic potential in atherosclerosis. Drs. Charles Dotter and Melvin Judkins performed the first peripheral angioplasty in a patient that had presented with gangrene and did not wish to have her leg amputated. Rigid polyethylene catheters were used to dilate the vessel; this intervention saved the patient’s leg [2].
About a decade later, Dr. Andreas Gruentzig, a cardiology fellow, performed the first human coronary angioplasty on an awake patient in Zurich, Germany in 1977; the patient was Mr. Dolf Bachmann, then a 38 year old patient with angina pectoris. This brought upon the advent of revolutionizing nonsurgical methods for revascularization. Initially, the concept of coronary angiography and then angioplasty received significant criticism and skepticism. After witnessing Dr. Gruentzig’s success, only then did the medical community realize the impact that percutaneous revascularization had on the treatment of coronary artery disease. Despite the success of the procedure, the equipment that was used consisted of 9F guiding catheters that were difficult to manipulate, balloons that ruptured quickly, and guidewires that could not be separated from the catheter. Early in 1980s, newer technology brought improved equipment for coronary angioplasty. By this time, Dr. Gruentzig had brought his ideas to the Emory Hospital in Atlanta, GA, completing 2500 angioplasties within 5 years. At the age of 46, Dr. Gruentzig passed in a tragic plane crash, leaving behind novel ideas of advancing interventional cardiology: laser angioplasty, stents, atherectomy and intracoronary ultrasounds.
In the years that ensued, the visionary ideas of Dr. Gruentzig became reality. Many interventional devices, including rotational atherectomy, lasers and stents, were invented, improved and promptly put in clinical use, thereby revolutionizing interventional cardiology. By 1997, more than one million angioplasties had been performed worldwide. Over the years, the extent of technological advances has refined this procedure that was once thought to be but impossible in humans. Last year (2017) marked the 40th anniversary of coronary angioplasty, which currently enjoys a first line recommendation for the treatment of stable coronary artery disease and acute coronary syndrome [3].
References:
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
About a decade later, Dr. Andreas Gruentzig, a cardiology fellow, performed the first human coronary angioplasty on an awake patient in Zurich, Germany in 1977; the patient was Mr. Dolf Bachmann, then a 38 year old patient with angina pectoris. This brought upon the advent of revolutionizing nonsurgical methods for revascularization. Initially, the concept of coronary angiography and then angioplasty received significant criticism and skepticism. After witnessing Dr. Gruentzig’s success, only then did the medical community realize the impact that percutaneous revascularization had on the treatment of coronary artery disease. Despite the success of the procedure, the equipment that was used consisted of 9F guiding catheters that were difficult to manipulate, balloons that ruptured quickly, and guidewires that could not be separated from the catheter. Early in 1980s, newer technology brought improved equipment for coronary angioplasty. By this time, Dr. Gruentzig had brought his ideas to the Emory Hospital in Atlanta, GA, completing 2500 angioplasties within 5 years. At the age of 46, Dr. Gruentzig passed in a tragic plane crash, leaving behind novel ideas of advancing interventional cardiology: laser angioplasty, stents, atherectomy and intracoronary ultrasounds.
In the years that ensued, the visionary ideas of Dr. Gruentzig became reality. Many interventional devices, including rotational atherectomy, lasers and stents, were invented, improved and promptly put in clinical use, thereby revolutionizing interventional cardiology. By 1997, more than one million angioplasties had been performed worldwide. Over the years, the extent of technological advances has refined this procedure that was once thought to be but impossible in humans. Last year (2017) marked the 40th anniversary of coronary angioplasty, which currently enjoys a first line recommendation for the treatment of stable coronary artery disease and acute coronary syndrome [3].
References:
- Mueller RL, Sanborn TA. The of : cardiac catheterization, angioplasty, and related interventions. Am Heart J. 1995 Jan;129(1):146-72.
- Bourassa MG. The history of cardiac catheterization. Can J Cardiol. 2005 Oct;21(12):1011-4.
- Braunwald E. The Simon Dack lecture. Cardiology: the past, the present, and the future. J Am Coll Cardiol. 2003 Dec 17;42(12):2031-41.
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Circle of Vieussens: Got Collaterals?
Description
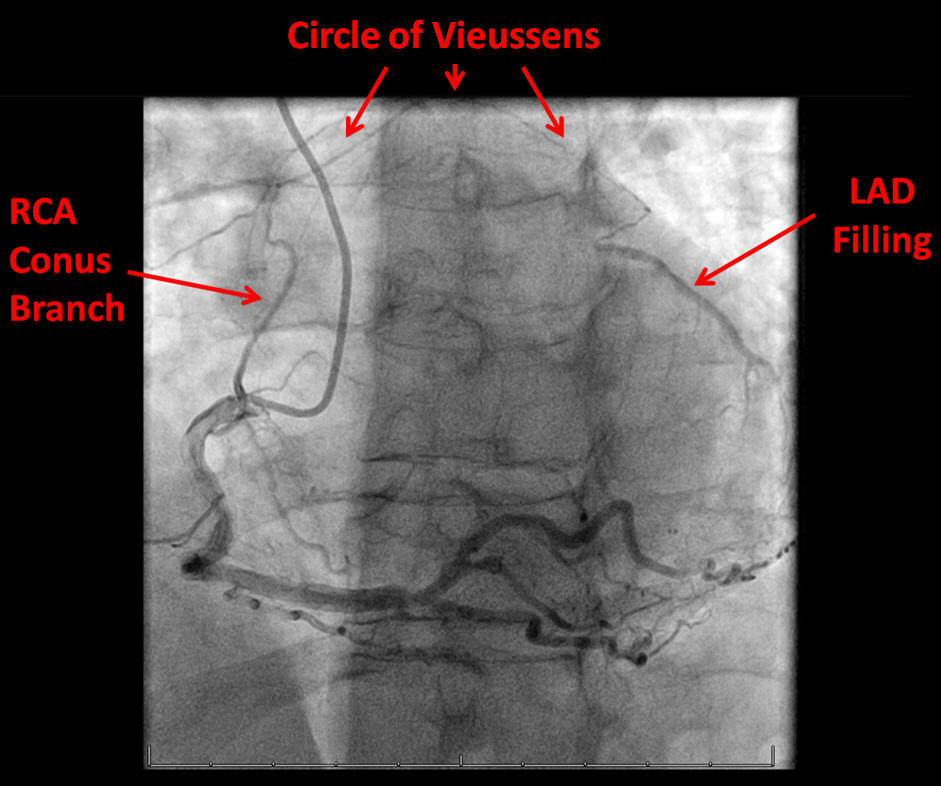
Circle of Vieussens [1] is an important coronary collateral pathway providing flow from the right coronary artery (RCA), via the conus artery, to the left anterior descending artery (LAD). It is seen in 20% of patients with total occlusion of the LAD. It can occur from stenotic lesions in either RCA or LAD. It was first described in 1706 by the French anatomist Raymond de Vieussens [2]. Coronary collateral circulation supply to totally occluded vessels plays a crucial role in the pathophysiology of coronary artery disease and is a strong predictor of symptoms and outcomes [3].
References
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Circle of Vieussens [1] is an important coronary collateral pathway providing flow from the right coronary artery (RCA), via the conus artery, to the left anterior descending artery (LAD). It is seen in 20% of patients with total occlusion of the LAD. It can occur from stenotic lesions in either RCA or LAD. It was first described in 1706 by the French anatomist Raymond de Vieussens [2]. Coronary collateral circulation supply to totally occluded vessels plays a crucial role in the pathophysiology of coronary artery disease and is a strong predictor of symptoms and outcomes [3].
References
- Deora S, Shah S, Patel T. "Arterial circle of Vieussens" - An important intercoronary collateral. Int J Cardiol Heart Vessel. 2014 Mar 6;3:84-85.
- Heinemann MK. A vicious Vieussens' circle? Thorac Cardiovasc Surg. 2011 Oct;59(7):385.
- Vo MN, Brilakis ES, Kass M, Ravandi A. Physiologic significance of coronary collaterals in chronic total occlusions. Can J Physiol Pharmacol. 2015 Oct;93(10):867-71.
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
Challenging Images
Kommerell's Diverticulum: Anomalous Anomaly!
Description
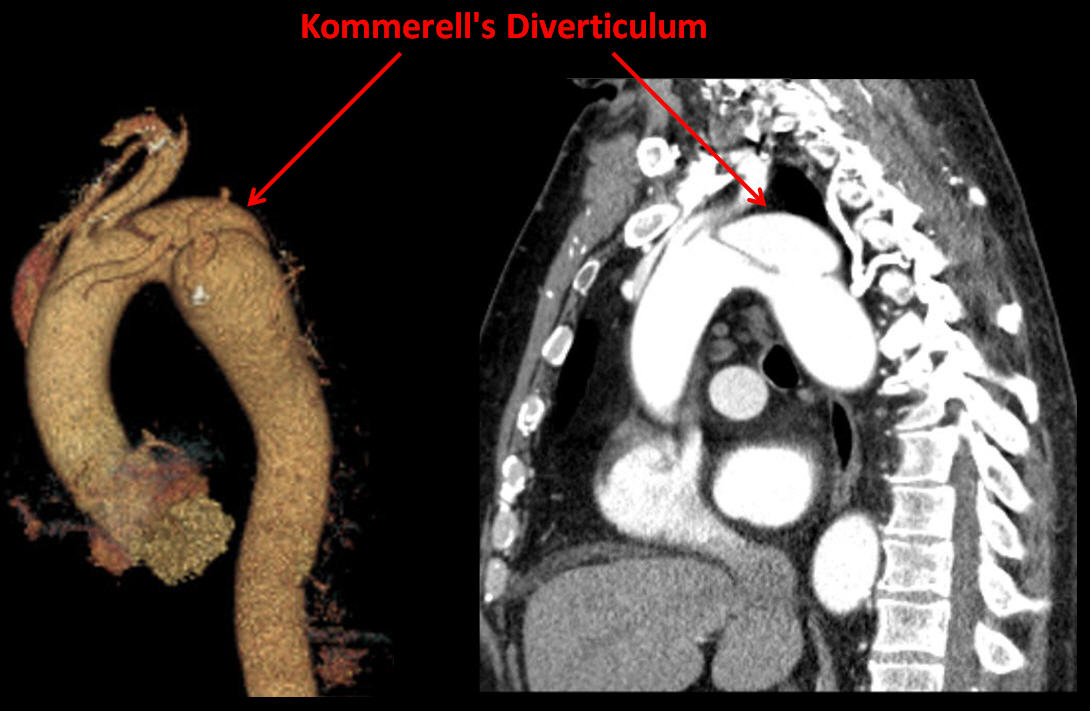
Kommerell’s diverticulum is an anomalous aneurysmal dilatation of the descending thoracic aorta, often associated with an anomalous origin of the right subclavian artery which has been reported in 0.4% to 2.0% of thoracic angiographic studies; other less common configurations, including anomalous left subclavian artery, have been described in the literature [1].
It can be present in 20-60% of patients with an aberrant subclavian artery, and has the potential to cause aneurysmal dilation, rupture or dissection of the aorta if left untreated. It is symptomatic in greater than 60% of patients; most common presenting symptoms include dysphagia and esophageal spasms, in addition to disordered sleep, dyspnea, atypical chest pain, and cough [2].
Treatment options, based on anatomy, center and operator experience, and other comorbid conditions, include surgical repair, hybrid surgical – endovascular repair, or complete endovascular intervention when the diameter of the diverticulum orifice is > 3.0 cm or adjacent aortic diameter is > 5.0 cm [3]. Kommerell’s diverticulum was described by Burckhard Kommerell, a German radiologist, in 1936 [4].
References
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Umer Awan, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL
Kommerell’s diverticulum is an anomalous aneurysmal dilatation of the descending thoracic aorta, often associated with an anomalous origin of the right subclavian artery which has been reported in 0.4% to 2.0% of thoracic angiographic studies; other less common configurations, including anomalous left subclavian artery, have been described in the literature [1].
It can be present in 20-60% of patients with an aberrant subclavian artery, and has the potential to cause aneurysmal dilation, rupture or dissection of the aorta if left untreated. It is symptomatic in greater than 60% of patients; most common presenting symptoms include dysphagia and esophageal spasms, in addition to disordered sleep, dyspnea, atypical chest pain, and cough [2].
Treatment options, based on anatomy, center and operator experience, and other comorbid conditions, include surgical repair, hybrid surgical – endovascular repair, or complete endovascular intervention when the diameter of the diverticulum orifice is > 3.0 cm or adjacent aortic diameter is > 5.0 cm [3]. Kommerell’s diverticulum was described by Burckhard Kommerell, a German radiologist, in 1936 [4].
References
- Fisher RG, Whigham CJ, Trinh C. Diverticula of Kommerell and aberrant subclavian arteries complicated by aneurysms. Cardiovasc Intervent Radiol. 2005 Sep-Oct;28(5):553-60.
- Poterucha J, Anavekar N, Niaz T, et al. Incidence and clinical presentation of Kommerell diverticulum and aneurysm. JACC March 17, 2015. Volume 65, Issue 10S. A524.
- van Son JA, Konstantinov IE. Burckhard F. Kommerell and Kommerell's diverticulum. Tex Heart Inst J. 2002;29(2):109-12.
- Tanaka A, Milner R, Ota T. Kommerell's diverticulum in the current era: a comprehensive review. Gen Thorac Cardiovasc Surg. 2015 May;63(5):245-59.
Authors:
Sarina Sachdev, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Hassan Tahir, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Muhammad Umer Awan, M.D.
Cardiology Fellow
University of South Alabama
Mobile, AL
Landai Nguyen, D.O.
Cardiology Fellow
University of South Alabama
Mobile, AL
Bassam Omar, M.D., Ph.D.
Professor of Cardiology
University of South Alabama
Mobile, AL
G. Mustafa Awan, M.D.
Associate Professor of Cardiology
University of South Alabama
Mobile, AL